Impact of Nisin and Nisin-Producing Lactococcus lactis ssp. lactis on Clostridium tyrobutyricum and Bacterial Ecosystem of Cheese Matrices
- PMID: 33921812
- PMCID: PMC8073774
- DOI: 10.3390/foods10040898
Impact of Nisin and Nisin-Producing Lactococcus lactis ssp. lactis on Clostridium tyrobutyricum and Bacterial Ecosystem of Cheese Matrices
Abstract
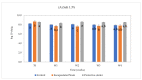
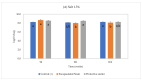
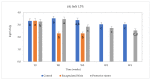
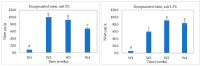
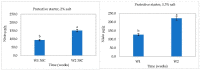
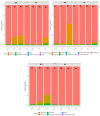
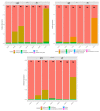
Clostridium tyrobutyricum spores survive milk pasteurization and cause late blowing of cheeses and significant economic loss. The effectiveness of nisin-producing Lactococcus lactis ssp. lactis 32 as a protective strain for control the C. tyrobutyricum growth in Cheddar cheese slurry was compared to that of encapsulated nisin-A. The encapsulated nisin was more effective, with 1.0 log10 reductions of viable spores after one week at 30 °C and 4 °C. Spores were not detected for three weeks at 4 °C in cheese slurry made with 1.3% salt, or during week 2 with 2% salt. Gas production was observed after one week at 30 °C only in the control slurry made with 1.3% salt. In slurry made with the protective strain, the reduction in C. tyrobutyricum count was 0.6 log10 in the second week at 4 °C with both salt concentration. At 4 °C, nisin production started in week 2 and reached 97 µg/g after four weeks. Metabarcoding analysis targeting the sequencing of 16S rRNA revealed that the genus Lactococcus dominated for four weeks at 4 °C. In cheese slurry made with 2% salt, the relative abundance of the genus Clostridium decreased significantly in the presence of nisin or the protective strain. The results indicated that both strategies are able to control the growth of Clostridium development in Cheddar cheese slurries.
Keywords: Cheddar cheese slurry; Clostridium tyrobutyricum; Lactococcus lactis; Nisin; antimicrobial peptides; dairy products; protective starter.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Bermúdez J., González M.J., Olivera J.A., Burgueño J.A., Juliano P., Fox E.M., Reginensi S.M. Seasonal occurrence and molecular diversity of clostridia species spores along cheesemaking streams of 5 commercial dairy plants. J. Dairy Sci. 2016;99:3358–3366. doi: 10.3168/jds.2015-10079. - DOI - PubMed
-
- Gómez-Torres N., Garde S., Peirotén Á., Ávila M. Impact of Clostridium spp. on cheese characteristics: Microbiology, color, formation of volatile compounds and off-flavors. Food Control. 2015;56:186–194.
-
- McSweeney P., Fox P. Cheese: Chemistry, Physics, and Microbiology. Volume 1. Elsevier; Amsterdam, The Netherlands: 2004. Metabolism of residual lactose and of lactate and citrate; pp. 361–371.
-
- Klijn N., Nieuwenhof F., Hoolwerf J.D., van der Waals C., Weerkamp A.H. Identification of Clostridium tyrobutyricum as the causative agent of late blowing in cheese by species-specific PCR amplification. Appl. Environ. Microbiol. 1995;61:2919–2924. doi: 10.1128/AEM.61.8.2919-2924.1995. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

