Noise-Induced Vascular Dysfunction, Oxidative Stress, and Inflammation Are Improved by Pharmacological Modulation of the NRF2/HO-1 Axis
- PMID: 33921821
- PMCID: PMC8073373
- DOI: 10.3390/antiox10040625
Noise-Induced Vascular Dysfunction, Oxidative Stress, and Inflammation Are Improved by Pharmacological Modulation of the NRF2/HO-1 Axis
Abstract
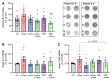
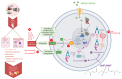
Vascular oxidative stress, inflammation, and subsequent endothelial dysfunction are consequences of traditional cardiovascular risk factors, all of which contribute to cardiovascular disease. Environmental stressors, such as traffic noise and air pollution, may also facilitate the development and progression of cardiovascular and metabolic diseases. In our previous studies, we investigated the influence of aircraft noise exposure on molecular mechanisms, identifying oxidative stress and inflammation as central players in mediating vascular function. The present study investigates the role of heme oxygenase-1 (HO-1) as an antioxidant response preventing vascular consequences following exposure to aircraft noise. C57BL/6J mice were treated with the HO-1 inducer hemin (25 mg/kg i.p.) or the NRF2 activator dimethyl fumarate (DMF, 20 mg/kg p.o.). During therapy, the animals were exposed to noise at a maximum sound pressure level of 85 dB(A) and a mean sound pressure level of 72 dB(A). Our data showed a marked protective effect of both treatments on animals exposed to noise for 4 days by normalization of arterial hypertension and vascular dysfunction in the noise-exposed groups. We observed a partial normalization of noise-triggered oxidative stress and inflammation by hemin and DMF therapy, which was associated with HO-1 induction. The present study identifies possible new targets for the mitigation of the adverse health effects caused by environmental noise exposure. Since natural dietary constituents can achieve HO-1 and NRF2 induction, these pathways represent promising targets for preventive measures.
Keywords: NRF2; aircraft noise exposure; endothelial dysfunction; environmental risk factors; heme oxygenase-1; inflammation; oxidative stress.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures






Similar articles
-
Mitigation of aircraft noise-induced vascular dysfunction and oxidative stress by exercise, fasting, and pharmacological α1AMPK activation: molecular proof of a protective key role of endothelial α1AMPK against environmental noise exposure.Eur J Prev Cardiol. 2023 Oct 26;30(15):1554-1568. doi: 10.1093/eurjpc/zwad075. Eur J Prev Cardiol. 2023. PMID: 37185661
-
Exacerbation of adverse cardiovascular effects of aircraft noise in an animal model of arterial hypertension.Redox Biol. 2020 Jul;34:101515. doi: 10.1016/j.redox.2020.101515. Epub 2020 Apr 18. Redox Biol. 2020. PMID: 32345536 Free PMC article.
-
Crucial role for Nox2 and sleep deprivation in aircraft noise-induced vascular and cerebral oxidative stress, inflammation, and gene regulation.Eur Heart J. 2018 Oct 7;39(38):3528-3539. doi: 10.1093/eurheartj/ehy333. Eur Heart J. 2018. PMID: 29905797 Free PMC article.
-
Oxidative stress and inflammation contribute to traffic noise-induced vascular and cerebral dysfunction via uncoupling of nitric oxide synthases.Redox Biol. 2020 Jul;34:101506. doi: 10.1016/j.redox.2020.101506. Epub 2020 Apr 20. Redox Biol. 2020. PMID: 32371009 Free PMC article. Review.
-
Environmental noise induces the release of stress hormones and inflammatory signaling molecules leading to oxidative stress and vascular dysfunction-Signatures of the internal exposome.Biofactors. 2019 Jul;45(4):495-506. doi: 10.1002/biof.1506. Epub 2019 Apr 2. Biofactors. 2019. PMID: 30937979 Review.
Cited by
-
Dimethyl Fumarate Prevents the Development of Chronic Social Stress-Induced Hypertension in Borderline Hypertensive Rats.Antioxidants (Basel). 2024 Aug 3;13(8):947. doi: 10.3390/antiox13080947. Antioxidants (Basel). 2024. PMID: 39199192 Free PMC article.
-
Vascular Redox Signaling, Endothelial Nitric Oxide Synthase Uncoupling, and Endothelial Dysfunction in the Setting of Transportation Noise Exposure or Chronic Treatment with Organic Nitrates.Antioxid Redox Signal. 2023 May;38(13-15):1001-1021. doi: 10.1089/ars.2023.0006. Epub 2023 Apr 6. Antioxid Redox Signal. 2023. PMID: 36719770 Free PMC article. Review.
-
Redox Switches in Noise-Induced Cardiovascular and Neuronal Dysregulation.Front Mol Biosci. 2021 Nov 18;8:784910. doi: 10.3389/fmolb.2021.784910. eCollection 2021. Front Mol Biosci. 2021. PMID: 34869603 Free PMC article. Review.
-
Environmental risk factors and cardiovascular diseases: a comprehensive expert review.Cardiovasc Res. 2022 Nov 10;118(14):2880-2902. doi: 10.1093/cvr/cvab316. Cardiovasc Res. 2022. PMID: 34609502 Free PMC article.
-
The Pharmacological Effect of Hemin in Inflammatory-Related Diseases: A Systematic Review.Biomedicines. 2024 Apr 18;12(4):898. doi: 10.3390/biomedicines12040898. Biomedicines. 2024. PMID: 38672251 Free PMC article. Review.
References
-
- Kalsch H., Hennig F., Moebus S., Mohlenkamp S., Dragano N., Jakobs H., Memmesheimer M., Erbel R., Jockel K.H., Hoffmann B., et al. Are air pollution and traffic noise independently associated with atherosclerosis: The heinz nixdorf recall study. Eur. Heart J. 2014;35:853–860. doi: 10.1093/eurheartj/eht426. - DOI - PubMed
-
- Schmidt F., Kolle K., Kreuder K., Schnorbus B., Wild P., Hechtner M., Binder H., Gori T., Munzel T. Nighttime aircraft noise impairs endothelial function and increases blood pressure in patients with or at high risk for coronary artery disease. Clin. Res. Cardiol. 2015;104:23–30. doi: 10.1007/s00392-014-0751-x. - DOI - PMC - PubMed
-
- Munzel T., Daiber A., Steven S., Tran L.P., Ullmann E., Kossmann S., Schmidt F.P., Oelze M., Xia N., Li H., et al. Effects of noise on vascular function, oxidative stress, and inflammation: Mechanistic insight from studies in mice. Eur. Heart J. 2017;38:2838–2849. doi: 10.1093/eurheartj/ehx081. - DOI - PMC - PubMed
-
- Kroller-Schon S., Daiber A., Steven S., Oelze M., Frenis K., Kalinovic S., Heimann A., Schmidt F.P., Pinto A., Kvandova M., et al. Crucial role for nox2 and sleep deprivation in aircraft noise-induced vascular and cerebral oxidative stress, inflammation, and gene regulation. Eur. Heart J. 2018;39:3528–3539. doi: 10.1093/eurheartj/ehy333. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

