Role of Microbiota-Derived Extracellular Vesicles in Gut-Brain Communication
- PMID: 33921831
- PMCID: PMC8073592
- DOI: 10.3390/ijms22084235
Role of Microbiota-Derived Extracellular Vesicles in Gut-Brain Communication
Abstract
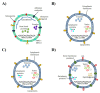
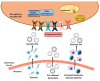
Human intestinal microbiota comprise of a dynamic population of bacterial species and other microorganisms with the capacity to interact with the rest of the organism and strongly influence the host during homeostasis and disease. Commensal and pathogenic bacteria coexist in homeostasis with the intestinal epithelium and the gastrointestinal tract's immune system, or GALT (gut-associated lymphoid tissue), of the host. However, a disruption to this homeostasis or dysbiosis by different factors (e.g., stress, diet, use of antibiotics, age, inflammatory processes) can cause brain dysfunction given the communication between the gut and brain. Recently, extracellular vesicles (EVs) derived from bacteria have emerged as possible carriers in gut-brain communication through the interaction of their vesicle components with immune receptors, which lead to neuroinflammatory immune response activation. This review discusses the critical role of bacterial EVs from the gut in the neuropathology of brain dysfunctions by modulating the immune response. These vesicles, which contain harmful bacterial EV contents such as lipopolysaccharide (LPS), peptidoglycans, toxins and nucleic acids, are capable of crossing tissue barriers including the blood-brain barrier and interacting with the immune receptors of glial cells (e.g., Toll-like receptors) to lead to the production of cytokines and inflammatory mediators, which can cause brain impairment and behavioral dysfunctions.
Keywords: bacteria; brain; extracellular vesicles; microbiota; neuropathology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Extracellular vesicles-mediated interaction within intestinal microenvironment in inflammatory bowel disease.J Adv Res. 2021 Jul 8;37:221-233. doi: 10.1016/j.jare.2021.07.002. eCollection 2022 Mar. J Adv Res. 2021. PMID: 35499059 Free PMC article. Review.
-
Host- and Microbiota-Derived Extracellular Vesicles, Immune Function, and Disease Development.Int J Mol Sci. 2019 Dec 22;21(1):107. doi: 10.3390/ijms21010107. Int J Mol Sci. 2019. PMID: 31877909 Free PMC article. Review.
-
Microbiota-Derived Extracellular Vesicle as Emerging Actors in Host Interactions.Int J Mol Sci. 2024 Aug 9;25(16):8722. doi: 10.3390/ijms25168722. Int J Mol Sci. 2024. PMID: 39201409 Free PMC article. Review.
-
Bacterial and eukaryotic extracellular vesicles and nonalcoholic fatty liver disease: new players in the gut-liver axis?Am J Physiol Gastrointest Liver Physiol. 2021 Apr 1;320(4):G485-G495. doi: 10.1152/ajpgi.00362.2020. Epub 2021 Jan 20. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33471632 Review.
-
Microbiota-derived extracellular vesicles in interkingdom communication in the gut.J Extracell Vesicles. 2021 Nov;10(13):e12161. doi: 10.1002/jev2.12161. J Extracell Vesicles. 2021. PMID: 34738337 Free PMC article. Review.
Cited by
-
Unraveling the Multifaceted Roles of Extracellular Vesicles: Insights into Biology, Pharmacology, and Pharmaceutical Applications for Drug Delivery.Int J Mol Sci. 2023 Dec 29;25(1):485. doi: 10.3390/ijms25010485. Int J Mol Sci. 2023. PMID: 38203656 Free PMC article. Review.
-
Bacterial extracellular vesicles - brain invaders? A systematic review.Front Mol Neurosci. 2023 Sep 14;16:1227655. doi: 10.3389/fnmol.2023.1227655. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37781094 Free PMC article.
-
Effects of gut microbiota-derived extracellular vesicles on obesity and diabetes and their potential modulation through diet.J Physiol Biochem. 2022 May;78(2):485-499. doi: 10.1007/s13105-021-00837-6. Epub 2021 Sep 2. J Physiol Biochem. 2022. PMID: 34472032 Free PMC article. Review.
-
Proteomic analysis of extracellular vesicles from tick hemolymph and uptake of extracellular vesicles by salivary glands and ovary cells.Parasit Vectors. 2023 Apr 13;16(1):125. doi: 10.1186/s13071-023-05753-w. Parasit Vectors. 2023. PMID: 37046327 Free PMC article.
-
Emerging role of microbiota derived outer membrane vesicles to preventive, therapeutic and diagnostic proposes.Infect Agent Cancer. 2023 Jan 19;18(1):3. doi: 10.1186/s13027-023-00480-4. Infect Agent Cancer. 2023. PMID: 36658631 Free PMC article. Review.
References
-
- Łuc M., Misiak B., Pawłowski M., Stańczykiewicz B., Zabłocka A., Szcześniak D., Pałęga A., Rymaszewska J. Gut microbiota in dementia. Critical review of novel findings and their potential application. Prog. Neuro Psychopharmacol. Biol. Psychiatry. 2021;104:110039. doi: 10.1016/j.pnpbp.2020.110039. - DOI - PubMed
-
- Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., Challis C., Schretter C.E., Rocha S., Gradinaru V., et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

