Synthesis of 3,4-Bis(Butylselanyl)Selenophenes and 4-Alkoxyselenophenes Promoted by Oxone®
- PMID: 33921844
- PMCID: PMC8073937
- DOI: 10.3390/molecules26082378
Synthesis of 3,4-Bis(Butylselanyl)Selenophenes and 4-Alkoxyselenophenes Promoted by Oxone®
Abstract
We describe herein an alternative transition-metal-free procedure to access 3,4-bis(butylselanyl)selenophenes and the so far unprecedented 3-(butylselanyl)-4-alkoxyselenophenes. The protocol involves the 5-endo-dig electrophilic cyclization of 1,3-diynes promoted by electrophilic organoselenium species, generated in situ through the oxidative cleavage of the Se-Se bond of dibutyl diselenide using Oxone® as a green oxidant. The selective formation of the title products was achieved by controlling the solvent identity and the amount of dibutyl diselenide. By using 4.0 equiv of dibutyl diselenide and acetonitrile as solvent at 80 °C, four examples of 3,4-bis(butylselanyl)selenophenes were obtained in moderate to good yields (40-78%). When 3.0 equiv of dibutyl diselenide were used, in the presence of aliphatic alcohols as solvent/nucleophiles under reflux, 10 3-(butylselanyl)-4-alkoxyselenophenes were selectively obtained in low to good yields (15-80%).
Keywords: 1,3-diynes; electrophilic cyclization; heterocycle; organoselenium; selenophene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

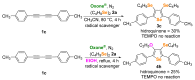

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

