In Vitro Investigation of Vascular Permeability in Endothelial Cells from Human Artery, Vein and Lung Microvessels at Steady-State and Anaphylactic Conditions
- PMID: 33921871
- PMCID: PMC8072631
- DOI: 10.3390/biomedicines9040439
In Vitro Investigation of Vascular Permeability in Endothelial Cells from Human Artery, Vein and Lung Microvessels at Steady-State and Anaphylactic Conditions
Abstract
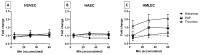
Human anaphylactic reactions largely involve an increase in vascular permeability, which is mainly controlled by endothelial cells (ECs). Due to the acute and serious nature of human anaphylaxis, in vivo studies of blood vessels must be replaced or supplemented with in vitro models. Therefore, we used a macromolecular tracer assay (MMTA) to investigate the EC permeability of three phenotypes of human ECs: artery (HAECs), vein (HSVECs) and microvessels from lung (HMLECs). ECs were stimulated with two fast-acting anaphylactic mediators (histamine and platelet-activating factor (PAF)) and one longer-lasting mediator (thrombin). At steady-state conditions, HSVEC monolayers were the most permeable and HMLEC the least (15.8% and 8.3% after 60 min, respectively). No response was found in ECs from artery or vein to any stimuli. ECs from microvessels reacted to stimulation with thrombin and also demonstrated a tendency of increased permeability for PAF. There was no reaction for histamine. This was not caused by missing receptor expression, as all three EC phenotypes expressed receptors for both PAF and histamine. The scarce response to fast-acting mediators illustrates that the MMTA is not suitable for investigating EC permeability to anaphylactic mediators.
Keywords: PAF; endothelial cells; histamine; macromolecular tracer assay; thrombin; vascular permeability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

