SARS-CoV-2 Mpro: A Potential Target for Peptidomimetics and Small-Molecule Inhibitors
- PMID: 33921886
- PMCID: PMC8073203
- DOI: 10.3390/biom11040607
SARS-CoV-2 Mpro: A Potential Target for Peptidomimetics and Small-Molecule Inhibitors
Abstract
The uncontrolled spread of the COVID-19 pandemic caused by the new coronavirus SARS-CoV-2 during 2020-2021 is one of the most devastating events in the history, with remarkable impacts on the health, economic systems, and habits of the entire world population. While some effective vaccines are nowadays approved and extensively administered, the long-term efficacy and safety of this line of intervention is constantly under debate as coronaviruses rapidly mutate and several SARS-CoV-2 variants have been already identified worldwide. Then, the WHO's main recommendations to prevent severe clinical complications by COVID-19 are still essentially based on social distancing and limitation of human interactions, therefore the identification of new target-based drugs became a priority. Several strategies have been proposed to counteract such viral infection, including the repurposing of FDA already approved for the treatment of HIV, HCV, and EBOLA, inter alia. Among the evaluated compounds, inhibitors of the main protease of the coronavirus (Mpro) are becoming more and more promising candidates. Mpro holds a pivotal role during the onset of the infection and its function is intimately related with the beginning of viral replication. The interruption of its catalytic activity could represent a relevant strategy for the development of anti-coronavirus drugs. SARS-CoV-2 Mpro is a peculiar cysteine protease of the coronavirus family, responsible for the replication and infectivity of the parasite. This review offers a detailed analysis of the repurposed drugs and the newly synthesized molecules developed to date for the treatment of COVID-19 which share the common feature of targeting SARS-CoV-2 Mpro, as well as a brief overview of the main enzymatic and cell-based assays to efficaciously screen such compounds.
Keywords: COVID-19; SARS-CoV-2 Mpro; coronavirus; peptidomimetics; protease inhibitors; remdesivir.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

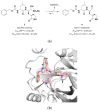
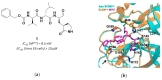
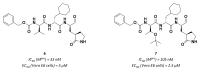
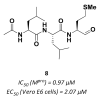

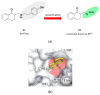

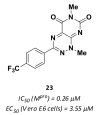
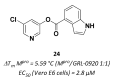
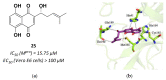
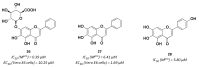
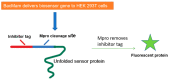
References
-
- Drosten C., Günter S., Preiser W., Van Der Weft S., Brodt H.-R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A.M., et al. Identification of a Novel Coronavirus in Patients with Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. - DOI - PubMed
-
- WHO Coronavirus (COVID-19) Dashboard. [(accessed on 26 March 2021)]; Available online: https://covid19.who.int/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

