Tumor Long Interspersed Nucleotide Element-1 (LINE-1) Hypomethylation in Relation to Age of Colorectal Cancer Diagnosis and Prognosis
- PMID: 33922024
- PMCID: PMC8122644
- DOI: 10.3390/cancers13092016
Tumor Long Interspersed Nucleotide Element-1 (LINE-1) Hypomethylation in Relation to Age of Colorectal Cancer Diagnosis and Prognosis
Abstract
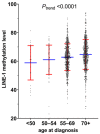
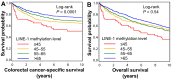
Evidence indicates the pathogenic role of epigenetic alterations in early-onset colorectal cancers diagnosed before age 50. However, features of colorectal cancers diagnosed at age 50-54 (hereafter referred to as "intermediate-onset") remain less known. We hypothesized that tumor long interspersed nucleotide element-1 (LINE-1) hypomethylation might be increasingly more common with decreasing age of colorectal cancer diagnosis. In 1356 colorectal cancers, including 28 early-onset and 66 intermediate-onset cases, the tumor LINE-1 methylation level measured by bisulfite-PCR-pyrosequencing (scaled 0 to 100) showed a mean of 63.6 (standard deviation (SD) 10.1). The mean tumor LINE-1 methylation level decreased with decreasing age (mean 64.7 (SD 10.4) in age ≥70, 62.8 (SD 9.4) in age 55-69, 61.0 (SD 10.2) in age 50-54, and 58.9 (SD 12.0) in age <50; p < 0.0001). In linear regression analysis, the multivariable-adjusted β coefficient (95% confidence interval (CI)) (vs. age ≥70) was -1.38 (-2.47 to -0.30) for age 55-69, -2.82 (-5.29 to -0.34) for age 50-54, and -4.54 (-8.24 to -0.85) for age <50 (Ptrend = 0.0003). Multivariable-adjusted hazard ratios (95% CI) for LINE-1 methylation levels of ≤45, 45-55, and 55-65 (vs. >65) were 2.33 (1.49-3.64), 1.39 (1.05-1.85), and 1.29 (1.02-1.63), respectively (Ptrend = 0.0005). In conclusion, tumor LINE-1 hypomethylation is increasingly more common with decreasing age of colorectal cancer diagnosis, suggesting a role of global DNA hypomethylation in colorectal cancer arising in younger adults.
Keywords: carcinogenesis; colorectal neoplasms; epigenomics; genomic instability; long interspersed nuclear element; molecular pathology; retrotransposon; screening; transposable element; young-onset cancer.
Conflict of interest statement
J.A.M. has received institutional research funding from Boston Biomedical, has served as an advisor/consultant to COTA Healthcare, and has served on a grant review panel for the National Comprehensive Cancer Network funded by Taiho Pharmaceutical. M.G. received research funding from Bristol-Myers Squibb, Merck, and Servier. This study was not funded by any of these commercial entities. A.T.C. previously served as a consultant for Bayer Healthcare and Pfizer Inc. This study was not funded by Bayer Healthcare or Pfizer Inc. K.N. has received institutional research funding from Evergrande Group, Genentech, Gilead Sciences, Pharmavite, Revolution Medicines, Tarrex Biopharma, and Trovagene and has served on advisory boards for Array Biopharma, Bayer, and Seattle Genetics. The other authors declare that they have no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

