A Breach in Plant Defences: Pseudomonas syringae pv. actinidiae Targets Ethylene Signalling to Overcome Actinidia chinensis Pathogen Responses
- PMID: 33922148
- PMCID: PMC8122719
- DOI: 10.3390/ijms22094375
A Breach in Plant Defences: Pseudomonas syringae pv. actinidiae Targets Ethylene Signalling to Overcome Actinidia chinensis Pathogen Responses
Abstract
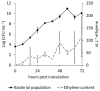
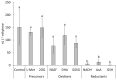
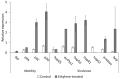
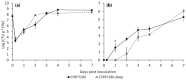
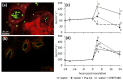
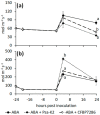
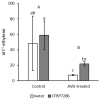
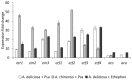
Ethylene interacts with other plant hormones to modulate many aspects of plant metabolism, including defence and stomata regulation. Therefore, its manipulation may allow plant pathogens to overcome the host's immune responses. This work investigates the role of ethylene as a virulence factor for Pseudomonas syringae pv. actinidiae (Psa), the aetiological agent of the bacterial canker of kiwifruit. The pandemic, highly virulent biovar of this pathogen produces ethylene, whereas the biovars isolated in Japan and Korea do not. Ethylene production is modulated in planta by light/dark cycle. Exogenous ethylene application stimulates bacterial virulence, and restricts or increases host colonisation if performed before or after inoculation, respectively. The deletion of a gene, unrelated to known bacterial biosynthetic pathways and putatively encoding for an oxidoreductase, abolishes ethylene production and reduces the pathogen growth rate in planta. Ethylene production by Psa may be a recently and independently evolved virulence trait in the arms race against the host. Plant- and pathogen-derived ethylene may concur in the activation/suppression of immune responses, in the chemotaxis toward a suitable entry point, or in the endophytic colonisation.
Keywords: bacterial canker of kiwifruit; plant hormones; plant immunity; stomata opening; virulence factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

