Small Bowel Epithelial Precursor Lesions: A Focus on Molecular Alterations
- PMID: 33922305
- PMCID: PMC8122855
- DOI: 10.3390/ijms22094388
Small Bowel Epithelial Precursor Lesions: A Focus on Molecular Alterations
Abstract
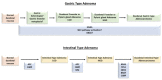
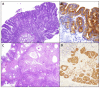
The wider use of gastrointestinal endoscopic procedures has led to an increased detection of small intestinal preneoplastic and neoplastic epithelial lesions, most of which are identified in the duodenum and ampullary region. Like their malignant counterparts, small intestinal glandular precursor lesions, which include adenomas and hamartomas, may arise sporadically or be associated with hereditary tumor syndromes, such as familial adenomatous polyposis, MUTYH-associated polyposis, Lynch syndrome, Peutz-Jeghers syndrome, juvenile polyposis syndrome, and Cowden syndrome. In addition, dysplastic, preinvasive lesions have been observed adjacent to small bowel adenocarcinomas complicating immune-related disorders, such as celiac or Crohn's disease. Adenomatous lesions may exhibit an intestinal-type, gastric-type, or, very rarely, serrated differentiation, related to different molecular pathogenetic mechanisms. Finally, in the background of multiple endocrine neoplasia 1 syndrome, precursor neuroendocrine growths have been described. In this review we offer a comprehensive description on the histo-molecular features of the main histotypes of small bowel epithelial precursors lesions, including: (i) sporadic adenomas (intestinal-type and gastric-type; non-ampullary and ampullary); (ii) syndromic adenomas; (iii) small bowel dysplasia in celiac and Crohn's disease; (iv) serrated lesions; (v) hamartomatous lesions; and (vi) neuroendocrine precursor lesions.
Keywords: APC; Crohn’s disease; GNAS; adenoma; ampulla; celiac disease; hamartoma; neuroendocrine; polyposis; small intestine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sekine S., Shia J. WHO Classification of Tumours Editorial Board. Digestive System Tumours. 5th ed. International Agency for Research on Cancer; Lyon, France: 2019. Non-ampullary adenoma; pp. 118–120.
-
- Okada K., Fujisaki J., Kasuga A., Omae M., Kubota M., Hirasawa T., Ishiyama A., Inamori M., Chino A., Yamamoto Y., et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: A study of follow-up surveillance. Am. J. Gastroenterol. 2011;106:357–364. doi: 10.1038/ajg.2010.422. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

