Inflammasomes in Teleosts: Structures and Mechanisms That Induce Pyroptosis during Bacterial Infection
- PMID: 33922312
- PMCID: PMC8122782
- DOI: 10.3390/ijms22094389
Inflammasomes in Teleosts: Structures and Mechanisms That Induce Pyroptosis during Bacterial Infection
Abstract
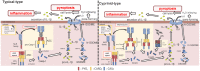
Pattern recognition receptors (PRRs) play a crucial role in inducing inflammatory responses; they recognize pathogen-associated molecular patterns, damage-associated molecular patterns, and environmental factors. Nucleotide-binding oligomerization domain-leucine-rich repeat-containing receptors (NLRs) are part of the PRR family; they form a large multiple-protein complex called the inflammasome in the cytosol. In mammals, the inflammasome consists of an NLR, used as a sensor molecule, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) as an adaptor protein, and pro-caspase1 (Casp1). Inflammasome activation induces Casp1 activation, promoting the maturation of proinflammatory cytokines, such as interleukin (IL)-1β and IL-18, and the induction of inflammatory cell death called pyroptosis via gasdermin D cleavage in mammals. Inflammasome activation and pyroptosis in mammals play important roles in protecting the host from pathogen infection. Recently, numerous inflammasome-related genes in teleosts have been identified, and their conservation and/or differentiation between their expression in mammals and teleosts have also been elucidated. In this review, we summarize the current knowledge of the molecular structure and machinery of the inflammasomes and the ASC-spec to induce pyroptosis; moreover, we explore the protective role of the inflammasome against pathogenic infection in teleosts.
Keywords: ASC; Caspase-1; NLR; gasdermin; inflammasome; pattern recognition; pyroptosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Inflammasomes and its importance in viral infections.Immunol Res. 2016 Dec;64(5-6):1101-1117. doi: 10.1007/s12026-016-8873-z. Immunol Res. 2016. PMID: 27699580 Review.
-
The zebrafish NLRP3 inflammasome has functional roles in ASC-dependent interleukin-1β maturation and gasdermin E-mediated pyroptosis.J Biol Chem. 2020 Jan 24;295(4):1120-1141. doi: 10.1074/jbc.RA119.011751. Epub 2019 Dec 18. J Biol Chem. 2020. PMID: 31852739 Free PMC article.
-
Caspase-1 interdomain linker cleavage is required for pyroptosis.Life Sci Alliance. 2020 Feb 12;3(3):e202000664. doi: 10.26508/lsa.202000664. Print 2020 Mar. Life Sci Alliance. 2020. PMID: 32051255 Free PMC article.
-
The pathological role of NLRs and AIM2 inflammasome-mediated pyroptosis in damaged blood-brain barrier after traumatic brain injury.Brain Res. 2018 Oct 15;1697:10-20. doi: 10.1016/j.brainres.2018.06.008. Epub 2018 Jun 8. Brain Res. 2018. PMID: 29886252
-
A Comparative Review of Pyroptosis in Mammals and Fish.J Inflamm Res. 2022 Apr 11;15:2323-2331. doi: 10.2147/JIR.S361266. eCollection 2022. J Inflamm Res. 2022. PMID: 35431566 Free PMC article. Review.
Cited by
-
A new type of Caspase-1 upon recognizing bacteria inhibits GSDME-dependent histone modification and NF-κB signaling.Commun Biol. 2025 May 29;8(1):827. doi: 10.1038/s42003-025-08290-7. Commun Biol. 2025. PMID: 40442231 Free PMC article.
-
CLEC5A Promotes Neuronal Pyroptosis in Rat Spinal Cord Injury Models by Interacting with TREM1 and Elevating NLRC4 Expression.eNeuro. 2024 Oct 25;11(10):ENEURO.0111-24.2024. doi: 10.1523/ENEURO.0111-24.2024. Print 2024 Oct. eNeuro. 2024. PMID: 39187376 Free PMC article.
-
Crosstalk among mitophagy, pyroptosis, ferroptosis, and necroptosis in central nervous system injuries.Neural Regen Res. 2024 Aug 1;19(8):1660-1670. doi: 10.4103/1673-5374.389361. Epub 2023 Nov 8. Neural Regen Res. 2024. PMID: 38103229 Free PMC article.
-
Immune gene variation associated with chromosome-scale differences among individual zebrafish genomes.Sci Rep. 2023 May 13;13(1):7777. doi: 10.1038/s41598-023-34467-3. Sci Rep. 2023. PMID: 37179373 Free PMC article.
-
A chromosome-level, haplotype-resolved genome assembly and annotation for the Eurasian minnow (Leuciscidae: Phoxinus phoxinus) provide evidence of haplotype diversity.Gigascience. 2025 Jan 6;14:giae116. doi: 10.1093/gigascience/giae116. Gigascience. 2025. PMID: 39877992 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

