Macrophages and Immune Responses in Uterine Fibroids
- PMID: 33922329
- PMCID: PMC8146588
- DOI: 10.3390/cells10050982
Macrophages and Immune Responses in Uterine Fibroids
Abstract
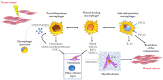
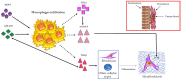
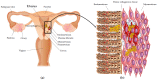
Uterine fibroids represent the most common benign tumors of the uterus. They are considered a typical fibrotic disorder. In fact, the extracellular matrix (ECM) proteins-above all, collagen 1A1, fibronectin and versican-are upregulated in this pathology. The uterine fibroids etiology has not yet been clarified, and this represents an important matter about their resolution. A model has been proposed according to which the formation of an altered ECM could be the result of an excessive wound healing, in turn driven by a dysregulated inflammation process. A lot of molecules act in the complex inflammatory response. Macrophages have a great flexibility since they can assume different phenotypes leading to the tissue repair process. The dysregulation of macrophage proliferation, accumulation and infiltration could lead to an uncontrolled tissue repair and to the consequent pathological fibrosis. In addition, molecules such as monocyte chemoattractant protein-1 (MCP-1), granulocyte macrophage-colony-stimulating factor (GM-CSF), transforming growth factor-beta (TGF-β), activin A and tumor necrosis factor-alfa (TNF-α) were demonstrated to play an important role in the macrophage action within the uncontrolled tissue repair that contributes to the pathological fibrosis that represents a typical feature of the uterine fibroids.
Keywords: ECM; inflammatory process; macrophages; pathological fibrosis; tissue repair; uterine fibroids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- McEvoy A., Sabir S. Physiology, Pregnancy Contractions. StatPearls; Treasure Island, FL, USA: 2021. - PubMed
-
- Valente R., Malesani M.G. Dizionario Medico. Larousse; Paris, France: 1984. p. 928.
-
- Gentile F. Enciclopedia Italiana. Volume 16. Grolier; New Delhi, India: 1987. p. 271.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

