Group Contribution Estimation of Ionic Liquid Melting Points: Critical Evaluation and Refinement of Existing Models
- PMID: 33922374
- PMCID: PMC8122861
- DOI: 10.3390/molecules26092454
Group Contribution Estimation of Ionic Liquid Melting Points: Critical Evaluation and Refinement of Existing Models
Abstract
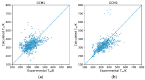
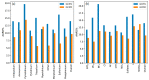
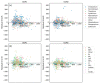
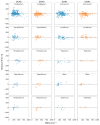
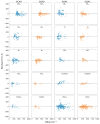
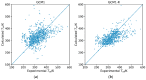
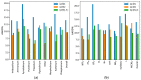
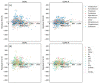
While several group contribution method (GCM) models have been developed in recent years for the prediction of ionic liquid (IL) properties, some challenges exist in their effective application. Firstly, the models have been developed and tested based on different datasets; therefore, direct comparison based on reported statistical measures is not reliable. Secondly, many of the existing models are limited in the range of ILs for which they can be used due to the lack of functional group parameters. In this paper, we examine two of the most diverse GCMs for the estimation of IL melting point; a key property in the selection and design of ILs for materials and energy applications. A comprehensive database consisting of over 1300 data points for 933 unique ILs, has been compiled and used to critically evaluate the two GCMs. One of the GCMs has been refined by introducing new functional groups and reparametrized to give improved performance for melting point estimation over a wider range of ILs. This work will aid in the targeted design of ILs for materials and energy applications.
Keywords: group contribution; ionic liquids; melting point; property estimation; thermal energy storage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ozokwelu D., Zhang S., Okafor O.C., Cheng W., Litombe N. Chapter 5—Separation Science and Technology. In: Ozokwelu D., Zhang S., Okafor O.C., Cheng W., Litombe N., editors. Novel Catalytic and Separation Processes Based on Ionic Liquids. Elsevier; Amsterdam, The Netherlands: 2017. pp. 193–202.
-
- Chen H., He Y., Zhu J., Alias H., Ding Y., Nancarrow P., Hardacre C., Rooney D., Tan C. Rheological and heat transfer behaviour of the ionic liquid, [C4mim][NTf2] Int. J. Heat Fluid Flow. 2008;29:149–155. doi: 10.1016/j.ijheatfluidflow.2007.05.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

