Anti-Tumor Effects of MAPK-Dependent Tumor-Selective Oncolytic Vaccinia Virus Armed with CD/UPRT against Pancreatic Ductal Adenocarcinoma in Mice
- PMID: 33922406
- PMCID: PMC8145488
- DOI: 10.3390/cells10050985
Anti-Tumor Effects of MAPK-Dependent Tumor-Selective Oncolytic Vaccinia Virus Armed with CD/UPRT against Pancreatic Ductal Adenocarcinoma in Mice
Abstract
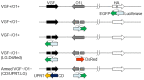
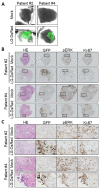
Engineered vaccinia virus serves as an oncolytic virus for cancer virotherapy. We evaluated the oncolytic characteristics of VGF- and O1-deleted recombinant mitogen-activated protein kinase (MAPK)-dependent vaccinia virus (MDRVV). We found that compared with viruses with the deletion of either gene alone, MDRVV is more attenuated in normal cells and can replicate in cancer cells that exhibit constitutive ERK1/2 activation in the MAPK pathway. We armed MDRVV with a bifunctional fusion gene encoding cytosine deaminase and uracil phosphoribosyltransferase (CD/UPRT), which converts 5-fluorocytosine (5-FC) into chemotherapeutic agents, and evaluated its oncolytic activity alone or in combination with 5-FC in human pancreatic cancer cell lines, tumor mouse models of peritoneal dissemination and liver metastasis, and ex vivo-infected live pancreatic cancer patient-derived tissues. CD/UPRT-armed MDRVV alone could efficiently eliminate pancreatic cancers, and its antitumor effects were partially enhanced in combination with 5-FC in vitro and in vivo. Moreover, the replication of MDRVV was detected in tumor cells of patient-derived, surgically resected tissues, which showed enlarged nuclei and high expression of pERK1/2 and Ki-67, and not in stromal cells. Our findings suggest that systemic injections of CD/UPRT-armed MDRVV alone or in combination with 5-FC are promising therapeutic strategies for pancreatic ductal adenocarcinoma.
Keywords: CD/UPRT; MAPK pathway; antitumor effects; oncolytic vaccinia virus; pancreatic cancer; prodrug 5-FC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Chemovirotherapy for head and neck squamous cell carcinoma with EGFR-targeted and CD/UPRT-armed oncolytic measles virus.Cancer Gene Ther. 2012 Mar;19(3):181-91. doi: 10.1038/cgt.2011.75. Epub 2011 Nov 11. Cancer Gene Ther. 2012. PMID: 22076043
-
Suicide gene therapy with the yeast fusion gene cytosine deaminase/uracil phosphoribosyltransferase is not enough for pancreatic cancer.Pancreas. 2007 Oct;35(3):224-31. doi: 10.1097/mpa.0b013e3180622519. Pancreas. 2007. PMID: 17895842
-
Placental mesenchymal stem cells: A promising platform for advancing gene therapy in pancreatic ductal adenocarcinoma.Biomed Pharmacother. 2025 Sep;190:118428. doi: 10.1016/j.biopha.2025.118428. Epub 2025 Aug 6. Biomed Pharmacother. 2025. PMID: 40768934
-
Gene therapy for pancreatic cancer targeting the genomic alterations of tumor suppressor genes using replication-selective oncolytic adenovirus.Hum Cell. 2002 Sep;15(3):138-50. doi: 10.1111/j.1749-0774.2002.tb00108.x. Hum Cell. 2002. PMID: 12703544 Review.
-
Vaccinia virus, a promising new therapeutic agent for pancreatic cancer.Immunotherapy. 2015;7(12):1249-58. doi: 10.2217/imt.15.90. Epub 2015 Nov 23. Immunotherapy. 2015. PMID: 26595180 Free PMC article. Review.
Cited by
-
Lens culinaris agglutinin inhibits human hepatoma cell migration via mannose and fucose-mediated ERK1/2 and JNK1/2/3 signalling pathway.Mol Biol Rep. 2022 Aug;49(8):7665-7676. doi: 10.1007/s11033-022-07582-z. Epub 2022 Jun 18. Mol Biol Rep. 2022. PMID: 35717475
-
Win or loss? Combination therapy does improve the oncolytic virus therapy to pancreatic cancer.Cancer Cell Int. 2022 Apr 20;22(1):160. doi: 10.1186/s12935-022-02583-1. Cancer Cell Int. 2022. PMID: 35443724 Free PMC article. Review.
-
Histone deacetylase inhibitor boosts anticancer potential of fusogenic oncolytic vaccinia virus by enhancing cell-cell fusion.Cancer Sci. 2024 Feb;115(2):600-610. doi: 10.1111/cas.16032. Epub 2023 Nov 30. Cancer Sci. 2024. PMID: 38037288 Free PMC article.
-
Tumor Tropism of DNA Viruses for Oncolytic Virotherapy.Viruses. 2023 Nov 16;15(11):2262. doi: 10.3390/v15112262. Viruses. 2023. PMID: 38005938 Free PMC article. Review.
-
Efficacy of Different Oncolytic Vaccinia Virus Strains for the Treatment of Murine Peritoneal Mesothelioma.Cancers (Basel). 2024 Jan 15;16(2):368. doi: 10.3390/cancers16020368. Cancers (Basel). 2024. PMID: 38254857 Free PMC article.
References
-
- McCart J.A., Ward J.M., Lee J., Hu Y., Alexander H.R., Libutti S.K., Moss B., Bartlett D.L. Systemic Cancer Therapy with a Tumor-Selective Vaccinia Virus Mutant Lacking Thymidine Kinase and Vaccinia Growth Factor Genes. Cancer Res. 2001;61:8751–8757. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

