Fucoidan from Laminaria japonica Inhibits Expression of GLUT9 and URAT1 via PI3K/Akt, JNK and NF-κB Pathways in Uric Acid-Exposed HK-2 Cells
- PMID: 33922488
- PMCID: PMC8145932
- DOI: 10.3390/md19050238
Fucoidan from Laminaria japonica Inhibits Expression of GLUT9 and URAT1 via PI3K/Akt, JNK and NF-κB Pathways in Uric Acid-Exposed HK-2 Cells
Abstract
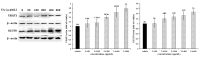
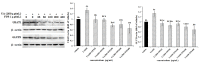
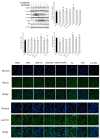
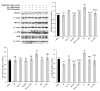
This work aimed to investigate the effect of fucoidan (FPS) on urate transporters induced by uric acid (UA). The results showed that UA stimulated the expression of glucose transporter 9 (GLUT9) and urate transporter 1 (URAT1) in HK-2 cells, and FPS could reverse the effect. Moreover, UA could activate NF-κB, JNK and PI3K/Akt pathways, but both pathway inhibitors and FPS inhibited the UA-induced activation of these three pathways. These data suggested that FPS effectively inhibited the expression induction of reabsorption transporters URAT1 and GLUT9 by UA, through repressing the activation of NF-κB, JNK and PI3K/Akt signal pathways in HK-2 cells. The in vitro research findings support the in vivo results that FPS reduces serum uric acid content in hyperuricemia mice and rats through inhibiting the expression of URAT1 and GLUT9 in renal tubular epithelial cells. This study provides a theoretical basis for the application of FPS in the treatment of hyperuricemia.
Keywords: fucoidan; glucose transporter 9; urate transporter 1; uric acid.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures

Similar articles
-
Fucoidan from Laminaria japonica protects renal tubular epithelial cells from uric acid induced NLRP3-mediated pyroptosis through inhibition of NF-κB pathway.J Ethnopharmacol. 2024 Dec 5;335:118614. doi: 10.1016/j.jep.2024.118614. Epub 2024 Jul 23. J Ethnopharmacol. 2024. PMID: 39053708
-
Renal Reabsorptive Transport of Uric Acid Precursor Xanthine by URAT1 and GLUT9.Biol Pharm Bull. 2020;43(11):1792-1798. doi: 10.1248/bpb.b20-00597. Biol Pharm Bull. 2020. PMID: 33132325
-
Urate transport capacity of glucose transporter 9 and urate transporter 1 in cartilage chondrocytes.Mol Med Rep. 2019 Aug;20(2):1645-1654. doi: 10.3892/mmr.2019.10426. Epub 2019 Jun 25. Mol Med Rep. 2019. PMID: 31257523 Free PMC article.
-
Uric acid metabolism in pre-hypertension and the metabolic syndrome.Curr Vasc Pharmacol. 2014;12(4):572-85. doi: 10.2174/1570161111999131205160756. Curr Vasc Pharmacol. 2014. PMID: 23627979 Review.
-
Renal transport of uric acid: evolving concepts and uncertainties.Adv Chronic Kidney Dis. 2012 Nov;19(6):358-71. doi: 10.1053/j.ackd.2012.07.009. Adv Chronic Kidney Dis. 2012. PMID: 23089270 Free PMC article. Review.
Cited by
-
Kaempferol attenuates hyperuricemia combined with gouty arthritis via urate transporters and NLRP3/NF-κB pathway modulation.iScience. 2024 Oct 16;27(11):111186. doi: 10.1016/j.isci.2024.111186. eCollection 2024 Nov 15. iScience. 2024. PMID: 39524334 Free PMC article.
-
α-Mangostin Attenuates Blood Pressure and Reverses Vascular Remodeling by Balancing ACE/AT1R and ACE2/Ang-(1-7)/MasR Axes in Ang II-Infused Hypertensive Mice.Phytother Res. 2024 Dec;38(12):5918-5929. doi: 10.1002/ptr.8353. Epub 2024 Oct 16. Phytother Res. 2024. PMID: 39410864 Free PMC article.
-
Expression Profile and Potential Function of Circular RNAs in Peripheral Blood Mononuclear Cells in Male Patients With Primary Gout.Front Genet. 2021 Oct 26;12:728091. doi: 10.3389/fgene.2021.728091. eCollection 2021. Front Genet. 2021. PMID: 34764979 Free PMC article.
-
Punicalagin attenuates hyperuricemia via restoring hyperuricemia-induced renal and intestinal dysfunctions.J Adv Res. 2025 Mar;69:449-461. doi: 10.1016/j.jare.2024.03.029. Epub 2024 Apr 10. J Adv Res. 2025. PMID: 38609050 Free PMC article.
-
Mining Xanthine Oxidase Inhibitors from an Edible Seaweed Pterocladiella capillacea by Using In Vitro Bioassays, Affinity Ultrafiltration LC-MS/MS, Metabolomics Tools, and In Silico Prediction.Mar Drugs. 2023 Sep 22;21(10):502. doi: 10.3390/md21100502. Mar Drugs. 2023. PMID: 37888437 Free PMC article.
References
-
- Chen W., Xiao Y. Hyperuricemia and its relative diseases. Chin. J. Lab. Diagn. 2016;20:2147–2150.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

