A Prototype Skin Substitute, Made of Recycled Marine Collagen, Improves the Skin Regeneration of Sheep
- PMID: 33922557
- PMCID: PMC8145883
- DOI: 10.3390/ani11051219
A Prototype Skin Substitute, Made of Recycled Marine Collagen, Improves the Skin Regeneration of Sheep
Abstract
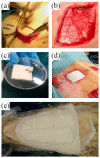
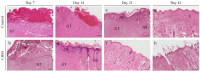
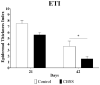
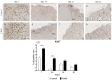
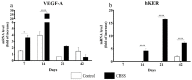
Skin wound healing is a complex and dynamic process that aims to restore lesioned tissues. Collagen-based skin substitutes are a promising treatment to promote wound healing by mimicking the native skin structure. Recently, collagen from marine organisms has gained interest as a source for producing biomaterials for skin regenerative strategies. This preliminary study aimed to describe the application of a collagen-based skin-like scaffold (CBSS), manufactured with collagen extracted from sea urchin food waste, to treat experimental skin wounds in a large animal. The wound-healing process was assessed over different time points by the means of clinical, histopathological, and molecular analysis. The CBSS treatment improved wound re-epithelialization along with cell proliferation, gene expression of growth factors (VEGF-A), and development of skin adnexa throughout the healing process. Furthermore, it regulated the gene expression of collagen type I and III, thus enhancing the maturation of the granulation tissue into a mature dermis without any signs of scarring as observed in untreated wounds. The observed results (reduced inflammation, better re-epithelialization, proper development of mature dermis and skin adnexa) suggest that sea urchin-derived CBSS is a promising biomaterial for skin wound healing in a "blue biotechnologies" perspective for animals of Veterinary interest.
Keywords: 3D scaffolds; biomaterials; circular economy; innovative therapies; marine collagen; regenerative medicine; sea urchin; skin substitute; tissue engineering; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


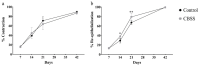


References
-
- Sparks H.D., Sigaeva T., Tarraf S., Mandla S., Pope H., Hee O., Di Martino E.S., Biernaskie J., Radisic M., Scott W.M. Biomechanics of Wound Healing in an Equine Limb Model: Effect of Location and Treatment with a Peptide-Modified Collagen-Chitosan Hydrogel. ACS Biomater. Sci. Eng. 2021;7:265–278. doi: 10.1021/acsbiomaterials.0c01431. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

