Triphenilphosphonium Analogs of Chloramphenicol as Dual-Acting Antimicrobial and Antiproliferating Agents
- PMID: 33922611
- PMCID: PMC8145938
- DOI: 10.3390/antibiotics10050489
Triphenilphosphonium Analogs of Chloramphenicol as Dual-Acting Antimicrobial and Antiproliferating Agents
Abstract
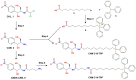
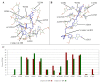
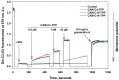
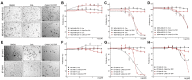
In the current work, in continuation of our recent research, we synthesized and studied new chimeric compounds, including the ribosome-targeting antibiotic chloramphenicol (CHL) and the membrane-penetrating cation triphenylphosphonium (TPP), which are linked by alkyl groups of different lengths. Using various biochemical assays, we showed that these CAM-Cn-TPP compounds bind to the bacterial ribosome, inhibit protein synthesis in vitro and in vivo in a way similar to that of the parent CHL, and significantly reduce membrane potential. Similar to CAM-C4-TPP, the mode of action of CAM-C10-TPP and CAM-C14-TPP in bacterial ribosomes differs from that of CHL. By simulating the dynamics of CAM-Cn-TPP complexes with bacterial ribosomes, we proposed a possible explanation for the specificity of the action of these analogs in the translation process. CAM-C10-TPP and CAM-C14-TPP more strongly inhibit the growth of the Gram-positive bacteria, as compared to CHL, and suppress some CHL-resistant bacterial strains. Thus, we have shown that TPP derivatives of CHL are dual-acting compounds targeting both the ribosomes and cellular membranes of bacteria. The TPP fragment of CAM-Cn-TPP compounds has an inhibitory effect on bacteria. Moreover, since the mitochondria of eukaryotic cells possess qualities similar to those of their prokaryotic ancestors, we demonstrate the possibility of targeting chemoresistant cancer cells with these compounds.
Keywords: alkyl(triphenyl)phosphonium; antibiotic activity; antiproliferative activity; bacterial ribosome; chloramphenicol; molecular dynamics simulations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Triphenylphosphonium Analogs of Short Peptide Related to Bactenecin 7 and Oncocin 112 as Antimicrobial Agents.Pharmaceutics. 2024 Jan 22;16(1):148. doi: 10.3390/pharmaceutics16010148. Pharmaceutics. 2024. PMID: 38276518 Free PMC article.
-
Conjugates of Chloramphenicol Amine and Berberine as Antimicrobial Agents.Antibiotics (Basel). 2022 Dec 22;12(1):15. doi: 10.3390/antibiotics12010015. Antibiotics (Basel). 2022. PMID: 36671216 Free PMC article.
-
Binding and Action of Triphenylphosphonium Analog of Chloramphenicol upon the Bacterial Ribosome.Antibiotics (Basel). 2021 Apr 5;10(4):390. doi: 10.3390/antibiotics10040390. Antibiotics (Basel). 2021. PMID: 33916420 Free PMC article.
-
Delocalized Lipophilic Cation Triphenyl Phosphonium: Promising Molecule for Mitochondria Targeting.Curr Drug Deliv. 2023;20(9):1217-1223. doi: 10.2174/1567201819666220525092527. Curr Drug Deliv. 2023. PMID: 35619273 Review.
-
The Mechanisms of Action of Ribosome-Targeting Peptide Antibiotics.Front Mol Biosci. 2018 May 14;5:48. doi: 10.3389/fmolb.2018.00048. eCollection 2018. Front Mol Biosci. 2018. PMID: 29868608 Free PMC article. Review.
Cited by
-
Streptomyces phaeochromogenes BV-204, K-1115A Anthraquinone-Producing Strain: A New Protein Biosynthesis Inhibitor.Acta Naturae. 2024 Jan-Mar;16(1):30-39. doi: 10.32607/actanaturae.27315. Acta Naturae. 2024. PMID: 38698962 Free PMC article.
-
Observation of Cytotoxicity of Phosphonium Derivatives Is Explained: Metabolism Inhibition and Adhesion Alteration.Antibiotics (Basel). 2023 Apr 6;12(4):720. doi: 10.3390/antibiotics12040720. Antibiotics (Basel). 2023. PMID: 37107081 Free PMC article.
-
Penetration of Triphenylphosphonium Derivatives through the Cell Envelope of Bacteria of Mycobacteriales Order.Pharmaceuticals (Basel). 2023 May 2;16(5):688. doi: 10.3390/ph16050688. Pharmaceuticals (Basel). 2023. PMID: 37242470 Free PMC article.
-
Triphenylphosphonium Analogs of Short Peptide Related to Bactenecin 7 and Oncocin 112 as Antimicrobial Agents.Pharmaceutics. 2024 Jan 22;16(1):148. doi: 10.3390/pharmaceutics16010148. Pharmaceutics. 2024. PMID: 38276518 Free PMC article.
-
Conjugates of Chloramphenicol Amine and Berberine as Antimicrobial Agents.Antibiotics (Basel). 2022 Dec 22;12(1):15. doi: 10.3390/antibiotics12010015. Antibiotics (Basel). 2022. PMID: 36671216 Free PMC article.
References
-
- Barbachyn M.R. Recent advances in the discovery of hybrid antibacterial agents. In: Macor J.E., editor. Annual Reports in Medicinal Chemistry. Elsevier Academic Press; San Diego, CA, USA: 2008. pp. 281–290. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

