Recent Progress in a Membrane-Based Technique for Propylene/Propane Separation
- PMID: 33922617
- PMCID: PMC8145504
- DOI: 10.3390/membranes11050310
Recent Progress in a Membrane-Based Technique for Propylene/Propane Separation
Abstract
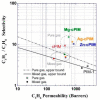
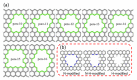
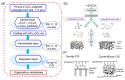
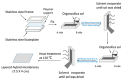
The similar physico-chemical properties of propylene and propane molecules have made the separation process of propylene/propane challenging. Membrane separation techniques show substantial prospects in propylene/propane separation due to their low energy consumption and investment costs, and they have been proposed to replace or to be combined with the conventional cryogenic distillation process. Over the past decade, organosilica membranes have attracted considerable attention due to their significant features, such as their good molecular sieving properties and high hydrothermal stability. In the present review, holistic insight is provided to summarize the recent progress in propylene/propane separation using polymeric, inorganic, and hybrid membranes, and a particular inspection of organosilica membranes is conducted. The importance of the pore subnano-environment of organosilica membranes is highlighted, and future directions and perspectives for propylene/propane separation are also provided.
Keywords: affinity control; hybrid membrane; inorganic membrane; organosilica membrane; polymeric membrane; pore size control; propylene/propane separation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Heteroepitaxially grown zeolitic imidazolate framework membranes with unprecedented propylene/propane separation performances.J Am Chem Soc. 2015 Sep 30;137(38):12304-11. doi: 10.1021/jacs.5b06730. Epub 2015 Sep 18. J Am Chem Soc. 2015. PMID: 26364888
-
Conformation-Controlled Molecular Sieving Effects for Membrane-Based Propylene/Propane Separation.Adv Mater. 2019 Apr;31(14):e1807513. doi: 10.1002/adma.201807513. Epub 2019 Feb 15. Adv Mater. 2019. PMID: 30768815
-
Pore configuration control in hybrid azolate ultra-microporous frameworks for sieving propylene from propane.Nat Chem. 2025 Jan;17(1):141-147. doi: 10.1038/s41557-024-01672-0. Epub 2024 Nov 15. Nat Chem. 2025. PMID: 39548209
-
Pore-Space Partition and Optimization for Propane-Selective High-Performance Propane/Propylene Separation.ACS Appl Mater Interfaces. 2021 Nov 10;13(44):52160-52166. doi: 10.1021/acsami.1c10391. Epub 2021 Jul 8. ACS Appl Mater Interfaces. 2021. PMID: 34236170 Review.
-
Zeolitic Imidazolate Framework Membranes: Novel Synthesis Methods and Progress Toward Industrial Use.Annu Rev Chem Biomol Eng. 2022 Jun 10;13:529-555. doi: 10.1146/annurev-chembioeng-092320-120148. Epub 2022 Apr 13. Annu Rev Chem Biomol Eng. 2022. PMID: 35417198 Review.
Cited by
-
Network Structure Engineering of Organosilica Membranes for Enhanced CO2 Capture Performance.Membranes (Basel). 2022 Apr 27;12(5):470. doi: 10.3390/membranes12050470. Membranes (Basel). 2022. PMID: 35629796 Free PMC article.
-
Pore size engineering of cost-effective all-nanoporous multilayer membranes for propane/propylene separation.Sci Rep. 2023 Dec 5;13(1):21419. doi: 10.1038/s41598-023-48841-8. Sci Rep. 2023. PMID: 38049544 Free PMC article.
-
Effect of Ultrasound on Dissolution of Polymeric Blends and Phase Inversion in Flat Sheet and Hollow Fiber Membranes for Ultrafiltration Applications.Membranes (Basel). 2025 Apr 10;15(4):120. doi: 10.3390/membranes15040120. Membranes (Basel). 2025. PMID: 40277990 Free PMC article.
-
Ultrasonically synthesized MOFs for modification of polymeric membranes: A critical review.Ultrason Sonochem. 2022 Nov;90:106202. doi: 10.1016/j.ultsonch.2022.106202. Epub 2022 Oct 14. Ultrason Sonochem. 2022. PMID: 36274415 Free PMC article. Review.
-
Design and Evaluation of Two-Stage Membrane-Separation Processes for Propylene-Propane Mixtures.Membranes (Basel). 2022 Jan 29;12(2):163. doi: 10.3390/membranes12020163. Membranes (Basel). 2022. PMID: 35207084 Free PMC article.
References
-
- Eldridge R.B. Olefin/paraffin separation technology: A review. Ind. Eng. Chem. Res. 1993;32:2208–2212. doi: 10.1021/ie00022a002. - DOI
-
- Nakayama N. Global Supply and Demand of Petrochemical Products Relied on LPG as Feedstock. International LP Gas Seminar; Tokyo, Japan: 2017.
-
- Ren T., Patel M., Blok K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy. 2006;31:425–451. doi: 10.1016/j.energy.2005.04.001. - DOI
-
- Faiz R., Li K. Olefin/paraffin separation using membrane based facilitated transport/chemical absorption techniques. Chem. Eng. Sci. 2012;73:261–284. doi: 10.1016/j.ces.2012.01.037. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

