Degeneration of Aortic Valves in a Bioreactor System with Pulsatile Flow
- PMID: 33922670
- PMCID: PMC8145810
- DOI: 10.3390/biomedicines9050462
Degeneration of Aortic Valves in a Bioreactor System with Pulsatile Flow
Abstract
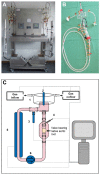
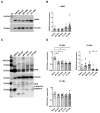
Calcific aortic valve disease is the most common valvular heart disease in industrialized countries. Pulsatile pressure, sheer and bending stress promote initiation and progression of aortic valve degeneration. The aim of this work is to establish an ex vivo model to study the therein involved processes. Ovine aortic roots bearing aortic valve leaflets were cultivated in an elaborated bioreactor system with pulsatile flow, physiological temperature, and controlled pressure and pH values. Standard and pro-degenerative treatment were studied regarding the impact on morphology, calcification, and gene expression. In particular, differentiation, matrix remodeling, and degeneration were also compared to a static cultivation model. Bioreactor cultivation led to shrinking and thickening of the valve leaflets compared to native leaflets while gross morphology and the presence of valvular interstitial cells were preserved. Degenerative conditions induced considerable leaflet calcification. In comparison to static cultivation, collagen gene expression was stable under bioreactor cultivation, whereas expression of hypoxia-related markers was increased. Osteopontin gene expression was differentially altered compared to protein expression, indicating an enhanced protein turnover. The present ex vivo model is an adequate and effective system to analyze aortic valve degeneration under controlled physiological conditions without the need of additional growth factors.
Keywords: ECM remodeling; bioreactor system; calcific aortic valve disease; degeneration; tissue cultivation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Crosstalk of Diabetic Conditions with Static Versus Dynamic Flow Environment-Impact on Aortic Valve Remodeling.Int J Mol Sci. 2021 Jun 28;22(13):6976. doi: 10.3390/ijms22136976. Int J Mol Sci. 2021. PMID: 34203572 Free PMC article.
-
Design of a cyclic pressure bioreactor for the ex vivo study of aortic heart valves.J Vis Exp. 2011 Aug 23;(54):3316. doi: 10.3791/3316. J Vis Exp. 2011. PMID: 21876532 Free PMC article.
-
Normal physiological conditions maintain the biological characteristics of porcine aortic heart valves: an ex vivo organ culture study.Ann Biomed Eng. 2005 Sep;33(9):1158-66. doi: 10.1007/s10439-005-5506-4. Ann Biomed Eng. 2005. PMID: 16133923
-
Hemodynamic and cellular response feedback in calcific aortic valve disease.Circ Res. 2013 Jul 5;113(2):186-97. doi: 10.1161/CIRCRESAHA.112.300154. Circ Res. 2013. PMID: 23833293 Review.
-
Founder's Award, 25th Annual Meeting of the Society for Biomaterials, perspectives. Providence, RI, April 28-May 2, 1999. Tissue heart valves: current challenges and future research perspectives.J Biomed Mater Res. 1999 Dec 15;47(4):439-65. doi: 10.1002/(sici)1097-4636(19991215)47:4<439::aid-jbm1>3.0.co;2-o. J Biomed Mater Res. 1999. PMID: 10497280 Review.
Cited by
-
Ex vivo model of pathological calcification of human aortic valve.Front Cardiovasc Med. 2024 Aug 29;11:1411398. doi: 10.3389/fcvm.2024.1411398. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39280032 Free PMC article.
-
Models for calcific aortic valve disease in vivo and in vitro.Cell Regen. 2024 Mar 1;13(1):6. doi: 10.1186/s13619-024-00189-8. Cell Regen. 2024. PMID: 38424219 Free PMC article. Review.
-
Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges.Biomedicines. 2022 May 8;10(5):1095. doi: 10.3390/biomedicines10051095. Biomedicines. 2022. PMID: 35625830 Free PMC article. Review.
-
Challenges of aortic valve tissue culture - maintenance of viability and extracellular matrix in the pulsatile dynamic microphysiological system.J Biol Eng. 2023 Sep 28;17(1):60. doi: 10.1186/s13036-023-00377-1. J Biol Eng. 2023. PMID: 37770970 Free PMC article.
-
Crosstalk of Diabetic Conditions with Static Versus Dynamic Flow Environment-Impact on Aortic Valve Remodeling.Int J Mol Sci. 2021 Jun 28;22(13):6976. doi: 10.3390/ijms22136976. Int J Mol Sci. 2021. PMID: 34203572 Free PMC article.
References
-
- Beckmann A., Funkat A.-K., Lewandowski J., Frie M., Ernst M., Hekmat K., Schiller W., Gummert J.F., Harringer W. German Heart Surgery Report 2016: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac. Cardiovasc. Surg. 2017;65:505–518. doi: 10.1055/s-0037-1606603. - DOI - PubMed
-
- Latif N., Sarathchandra P., Taylor P.M., Antoniw J., Yacoub M.H. Localization and pattern of expression of extracellular matrix components in human heart valves. J. Heart Valve Dis. 2005;14:218–227. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

