Accessible LAMP-Enabled Rapid Test (ALERT) for Detecting SARS-CoV-2
- PMID: 33922716
- PMCID: PMC8146324
- DOI: 10.3390/v13050742
Accessible LAMP-Enabled Rapid Test (ALERT) for Detecting SARS-CoV-2
Abstract
The coronavirus disease 2019 (COVID-19) pandemic has highlighted bottlenecks in large-scale, frequent testing of populations for infections. Polymerase chain reaction (PCR)-based diagnostic tests are expensive, reliant on centralized labs, can take days to deliver results, and are prone to backlogs and supply shortages. Antigen tests that bind and detect the surface proteins of a virus are rapid and scalable but suffer from high false negative rates. To address this problem, an inexpensive, simple, and robust 60-minute do-it-yourself (DIY) workflow to detect viral RNA from nasal swabs or saliva with high sensitivity (0.1 to 2 viral particles/μL) and specificity (>97% true negative rate) utilizing reverse transcription loop-mediated isothermal amplification (RT-LAMP) was developed. ALERT (Accessible LAMP-Enabled Rapid Test) incorporates the following features: (1) increased shelf-life and ambient temperature storage, compared to liquid reaction mixes, by using wax layers to isolate enzymes from other reagents; (2) improved specificity compared to other LAMP end-point reporting methods, by using sequence-specific QUASR (quenching of unincorporated amplification signal reporters); (3) increased sensitivity, compared to methods without purification through use of a magnetic wand to enable pipette-free concentration of sample RNA and cell debris removal; (4) quality control with a nasopharyngeal-specific mRNA target; and (5) co-detection of other respiratory viruses, such as influenza B, by multiplexing QUASR-modified RT-LAMP primer sets. The flexible nature of the ALERT workflow allows easy, at-home and point-of-care testing for individuals and higher-throughput processing for labs and hospitals. With minimal effort, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific primer sets can be swapped out for other targets to repurpose ALERT to detect other viruses, microorganisms, or nucleic acid-based markers.
Keywords: RT-LAMP; SARS-CoV-2; biodetection; point-of-care.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



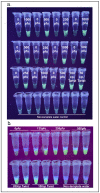

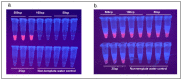

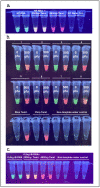
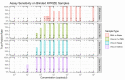
Similar articles
-
Extraction-free RT-LAMP to detect SARS-CoV-2 is less sensitive but highly specific compared to standard RT-PCR in 101 samples.J Clin Virol. 2021 Mar;136:104764. doi: 10.1016/j.jcv.2021.104764. Epub 2021 Feb 16. J Clin Virol. 2021. PMID: 33636553 Free PMC article.
-
Evaluation of self-collected nasal, urine, and saliva samples for molecular detection of SARS-CoV-2 using an EUA approved RT-PCR assay and a laboratory developed LAMP SARS-CoV-2 test.Immun Inflamm Dis. 2024 Jun;12(6):e1285. doi: 10.1002/iid3.1285. Immun Inflamm Dis. 2024. PMID: 38888444 Free PMC article.
-
Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection.mSphere. 2020 Aug 26;5(4):e00808-20. doi: 10.1128/mSphere.00808-20. mSphere. 2020. PMID: 32848011 Free PMC article.
-
A Recent Update on Advanced Molecular Diagnostic Techniques for COVID-19 Pandemic: An Overview.Front Immunol. 2021 Dec 14;12:732756. doi: 10.3389/fimmu.2021.732756. eCollection 2021. Front Immunol. 2021. PMID: 34970254 Free PMC article. Review.
-
Loop-mediated isothermal amplification (LAMP): An effective molecular point-of-care technique for the rapid diagnosis of coronavirus SARS-CoV-2.Rev Med Virol. 2021 Nov;31(6):e2215. doi: 10.1002/rmv.2215. Epub 2021 Jan 21. Rev Med Virol. 2021. PMID: 33476080 Free PMC article. Review.
Cited by
-
Evaluation of indirect sequence-specific magneto-extraction-aided LAMP for fluorescence and electrochemical SARS-CoV-2 nucleic acid detection.Talanta. 2023 Jan 15;252:123809. doi: 10.1016/j.talanta.2022.123809. Epub 2022 Aug 12. Talanta. 2023. PMID: 35985192 Free PMC article.
-
Rapid, Affordable, and Scalable SARS-CoV-2 Detection From Saliva.J Biomol Tech. 2021 Sep;32(3):148-157. doi: 10.7171/jbt.21-3203-010. J Biomol Tech. 2021. PMID: 35027872 Free PMC article.
-
Point-of-Care Testing for Hepatitis Viruses: A Growing Need.Life (Basel). 2023 Nov 28;13(12):2271. doi: 10.3390/life13122271. Life (Basel). 2023. PMID: 38137872 Free PMC article. Review.
-
Loop-Mediated Isothermal Amplification Detection of SARS-CoV-2 and Myriad Other Applications.J Biomol Tech. 2021 Sep;32(3):228-275. doi: 10.7171/jbt.21-3203-017. J Biomol Tech. 2021. PMID: 35136384 Free PMC article. Review.
-
Influenza A, Influenza B, and SARS-CoV-2 Similarities and Differences - A Focus on Diagnosis.Front Microbiol. 2022 Jun 20;13:908525. doi: 10.3389/fmicb.2022.908525. eCollection 2022. Front Microbiol. 2022. PMID: 35794916 Free PMC article. Review.
References
-
- Bhadra S., Maranhao A., Paik I., Ellington A. A one-enzyme RT-qPCR Assay for SARS-CoV-2, and Procedures for Reagent Production. Bioprotocol. 2021;11 doi: 10.21769/BioProtoc.3898. - DOI
-
- Mascuch S.J., Fakhretaha-Aval S., Bowman J.C., Ma M.T.H., Thomas G., Bommarius B., Ito C., Zhao L., Newnam G.P., Matange K.R., et al. A blueprint for academic laboratories to produce SARS-CoV-2 quantitative RT-PCR test kits. J. Biol. Chem. 2020;295:15438–15453. doi: 10.1074/jbc.RA120.015434. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

