Identification of TLR2 Signalling Mechanisms Which Contribute to Barrett's and Oesophageal Adenocarcinoma Disease Progression
- PMID: 33922955
- PMCID: PMC8123271
- DOI: 10.3390/cancers13092065
Identification of TLR2 Signalling Mechanisms Which Contribute to Barrett's and Oesophageal Adenocarcinoma Disease Progression
Abstract
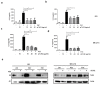
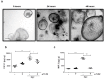
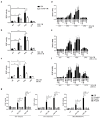
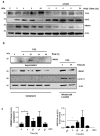
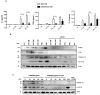
Chronic inflammation plays an important role in the pathogenesis of oesophageal adenocarcinoma (EAC) and its only known precursor, Barrett's oesophagus (BE). Recent studies have shown that oesophageal TLR2 levels increase from normal epithelium towards EAC. TLR2 signalling is therefore likely to be important during EAC development and progression, which requires an inflammatory microenvironment. Here, we show that, in response to TLR2 stimulation, BE organoids and early-stage EAC cells secrete pro-inflammatory cytokines and chemokines which recruit macrophages to the tumour site. Factors secreted from TLR2-stimulated EAC cells are shown to subsequently activate TLR2 on naïve macrophages, priming them for inflammasome activation and inducing their differentiation to an M2/TAM-like phenotype. We identify the endogenous TLR2 ligand, HMGB1, as the factor secreted from EAC cells responsible for the observed TLR2-mediated effects on macrophages. Our results indicate that HMGB1 signalling between EAC cells and macrophages creates an inflammatory tumour microenvironment to facilitate EAC progression. In addition to identifying HMGB1 as a potential target for early-stage EAC treatment, our data suggest that blocking TLR2 signalling represents a mechanism to limit HMGB1 release, inflammatory cell infiltration and inflammation during EAC progression.
Keywords: Barrett’s oesophagus; HMGB1; TLR2 signalling; inflammation; oesophageal adenocarcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

