Fiber-Templated 3D Calcium-Phosphate Scaffolds for Biomedical Applications: The Role of the Thermal Treatment Ambient on Physico-Chemical Properties
- PMID: 33922963
- PMCID: PMC8123353
- DOI: 10.3390/ma14092198
Fiber-Templated 3D Calcium-Phosphate Scaffolds for Biomedical Applications: The Role of the Thermal Treatment Ambient on Physico-Chemical Properties
Abstract
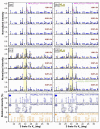
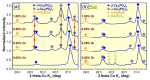
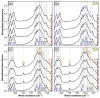
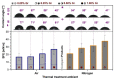
A successful bone-graft-controlled healing entails the development of novel products with tunable compositional and architectural features and mechanical performances and is, thereby, able to accommodate fast bone in-growth and remodeling. To this effect, graphene nanoplatelets and Luffa-fibers were chosen as mechanical reinforcement phase and sacrificial template, respectively, and incorporated into a hydroxyapatite and brushite matrix derived by marble conversion with the help of a reproducible technology. The bio-products, framed by a one-stage-addition polymer-free fabrication route, were thoroughly physico-chemically investigated (by XRD, FTIR spectroscopy, SEM, and nano-computed tomography analysis, as well as surface energy measurements and mechanical performance assessments) after sintering in air or nitrogen ambient. The experiments exposed that the coupling of a nitrogen ambient with the graphene admixing triggers, in both compact and porous samples, important structural (i.e., decomposition of β-Ca3(PO4)2 into α-Ca3(PO4)2 and α-Ca2P2O7) and morphological modifications. Certain restrictions and benefits were outlined with respect to the spatial porosity and global mechanical features of the derived bone scaffolds. Specifically, in nitrogen ambient, the graphene amount should be set to a maximum 0.25 wt.% in the case of compact products, while for the porous ones, significantly augmented compressive strengths were revealed at all graphene amounts. The sintering ambient or the graphene addition did not interfere with the Luffa ability to generate 3D-channels-arrays at high temperatures. It can be concluded that both Luffa and graphene agents act as adjuvants under nitrogen ambient, and that their incorporation-ratio can be modulated to favorably fit certain foreseeable biomedical applications.
Keywords: Luffa; applications; biomedical; graphene; marble; reinforced bio-products; sintering ambient.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Preliminary Studies on Graphene-Reinforced 3D Products Obtained by the One-Stage Sacrificial Template Method for Bone Reconstruction Applications.J Funct Biomater. 2021 Feb 12;12(1):13. doi: 10.3390/jfb12010013. J Funct Biomater. 2021. PMID: 33673093 Free PMC article.
-
Fabrication and in vitro characterization of luffa-based composite scaffolds incorporated with gelatin, hydroxyapatite and psyllium husk for bone tissue engineering.J Biomater Sci Polym Ed. 2022 Dec;33(17):2220-2248. doi: 10.1080/09205063.2022.2101415. Epub 2022 Jul 22. J Biomater Sci Polym Ed. 2022. PMID: 35820154
-
Enhanced sintering ability of biphasic calcium phosphate by polymers used for bone scaffold fabrication.Mater Sci Eng C Mater Biol Appl. 2013 Oct;33(7):3802-10. doi: 10.1016/j.msec.2013.05.017. Epub 2013 May 14. Mater Sci Eng C Mater Biol Appl. 2013. PMID: 23910280
-
3D Continuously Porous Graphene for Energy Applications.Adv Mater. 2022 Apr;34(15):e2108750. doi: 10.1002/adma.202108750. Epub 2022 Feb 25. Adv Mater. 2022. PMID: 34870863 Review.
-
Graphene Incorporated Electrospun Nanofiber for Electrochemical Sensing and Biomedical Applications: A Critical Review.Sensors (Basel). 2022 Nov 9;22(22):8661. doi: 10.3390/s22228661. Sensors (Basel). 2022. PMID: 36433257 Free PMC article. Review.
Cited by
-
Modulated Laser Cladding of Implant-Type Coatings by Bovine-Bone-Derived Hydroxyapatite Powder Injection on Ti6Al4V Substrates-Part I: Fabrication and Physico-Chemical Characterization.Materials (Basel). 2022 Nov 11;15(22):7971. doi: 10.3390/ma15227971. Materials (Basel). 2022. PMID: 36431457 Free PMC article.
-
Selection Route of Precursor Materials in 3D Printing Composite Filament Development for Biomedical Applications.Materials (Basel). 2023 Mar 15;16(6):2359. doi: 10.3390/ma16062359. Materials (Basel). 2023. PMID: 36984239 Free PMC article.
References
-
- De Groot K. Bioceramics of calcium phosphate. J. Clin. Eng. 1984;9:52. doi: 10.1097/00004669-198401000-00010. - DOI
-
- Mocanu A.-C., Miculescu F., Miculescu M., Ciocoiu R.C., Pandele A.M., Stan G.E., Cîmpean A., Voicu Ș.I., Ciocan L.-T. Comprehensive analysis of compatible natural fibre as sacrificial porogen template for tailored ceramic 3D bioproducts destined for hard tissue reconstruction. Ceram. Int. 2020;47:5318–5334. doi: 10.1016/j.ceramint.2020.10.113. - DOI
Grants and funding
- PN-III-P1-1.1-TE-2019-0463/Unitatea Executiva Pentru Finantarea Invatamantului Superior a Cercetarii Dezvoltarii si Inovarii
- PN-III-P1-1.2-PCCDI-2017-0062/contract no. 58PCCDI/2018/component project no. 2/Unitatea Executiva Pentru Finantarea Invatamantului Superior a Cercetarii Dezvoltarii si Inovarii
- institutional Core Program 21N/Unitatea Executiva Pentru Finantarea Invatamantului Superior a Cercetarii Dezvoltarii si Inovarii
LinkOut - more resources
Full Text Sources

