Platinum Complexes in Colorectal Cancer and Other Solid Tumors
- PMID: 33922989
- PMCID: PMC8123298
- DOI: 10.3390/cancers13092073
Platinum Complexes in Colorectal Cancer and Other Solid Tumors
Abstract
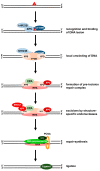
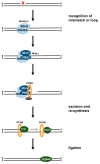
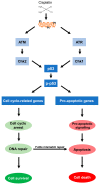
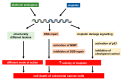
Cisplatin is one of the most commonly used drugs for the treatment of various solid neoplasms, including testicular, lung, ovarian, head and neck, and bladder cancers. Unfortunately, the therapeutic efficacy of cisplatin against colorectal cancer is poor. Various mechanisms appear to contribute to cisplatin resistance in cancer cells, including reduced drug accumulation, enhanced drug detoxification, modulation of DNA repair mechanisms, and finally alterations in cisplatin DNA damage signaling preventing apoptosis in cancer cells. Regarding colorectal cancer, defects in mismatch repair and altered p53-mediated DNA damage signaling are the main factors controlling the resistance phenotype. In particular, p53 inactivation appears to be associated with chemoresistance and poor prognosis. To overcome resistance in cancers, several strategies can be envisaged. Improved cisplatin analogues, which retain activity in resistant cancer, might be applied. Targeting p53-mediated DNA damage signaling provides another therapeutic strategy to circumvent cisplatin resistance. This review provides an overview on the DNA repair pathways involved in the processing of cisplatin damage and will describe signal transduction from cisplatin DNA lesions, with special attention given to colorectal cancer cells. Furthermore, examples for improved platinum compounds and biochemical modulators of cisplatin DNA damage signaling will be presented in the context of colon cancer therapy.
Keywords: colorectal cancer; mismatch repair defect; p53 signaling; platinum drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

