Ubiquitination, Biotech Startups, and the Future of TRIM Family Proteins: A TRIM-Endous Opportunity
- PMID: 33923045
- PMCID: PMC8146955
- DOI: 10.3390/cells10051015
Ubiquitination, Biotech Startups, and the Future of TRIM Family Proteins: A TRIM-Endous Opportunity
Abstract
Ubiquitination is a post-translational modification that has pivotal roles in protein degradation and diversified cellular processes, and for more than two decades it has been a subject of interest in the biotech or biopharmaceutical industry. Tripartite motif (TRIM) family proteins are known to have proven E3 ubiquitin ligase activities and are involved in a multitude of cellular and physiological events and pathophysiological conditions ranging from cancers to rare genetic disorders. Although in recent years many kinds of E3 ubiquitin ligases have emerged as the preferred choices of big pharma and biotech startups in the context of protein degradation and disease biology, from a surface overview it appears that TRIM E3 ubiquitin ligases are not very well recognized yet in the realm of drug discovery. This article will review some of the blockbuster scientific discoveries and technological innovations from the world of ubiquitination and E3 ubiquitin ligases that have impacted the biopharma community, from biotech colossuses to startups, and will attempt to evaluate the future of TRIM family proteins in the province of E3 ubiquitin ligase-based drug discovery.
Keywords: E3 ligases; TRIM family; drug discovery; startups; ubiquitination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

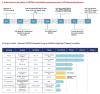
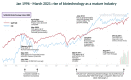
References
-
- Goldstein G., Scheid M., Hammerling U., Schlesinger D.H., Niall H.D., Boyse E.A. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc. Natl. Acad. Sci. USA. 1975;72:11–15. doi: 10.1073/pnas.72.1.11. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

