COVID-19 Vaccines: A Review of the Safety and Efficacy of Current Clinical Trials
- PMID: 33923054
- PMCID: PMC8144958
- DOI: 10.3390/ph14050406
COVID-19 Vaccines: A Review of the Safety and Efficacy of Current Clinical Trials
Abstract
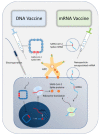
Various strategies have been designed to contain the COVID-19 pandemic. Among them, vaccine development is high on the agenda in spite of the unknown duration of the protection time. Various vaccines have been under clinical trials with promising results in different countries. The protective efficacy and the short-term and long-term side effects of the vaccines are of major concern. Therefore, comparing the protective efficacy and risks of vaccination is essential for the global control of COVID-19 through herd immunity. This study reviews the most recent data of 12 vaccines to evaluate their efficacy, safety profile and usage in various populations.
Keywords: COVID-19; efficacy; herd immunity; safety; vaccine.
Conflict of interest statement
The authors Zhipeng Yan, Ming Yang and Ching-Lung Lai declare there is no conflict of interest.
Figures
References
-
- World Health Organization [(accessed on 25 March 2021)];Weekly Epidemiology Update 23 February 2021. 2021 WHO Situation Report. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update---....
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

