Design and Characterization of Electrochemical Sensor for the Determination of Mercury(II) Ion in Real Samples Based upon a New Schiff Base Derivative as an Ionophore
- PMID: 33923078
- PMCID: PMC8123339
- DOI: 10.3390/s21093020
Design and Characterization of Electrochemical Sensor for the Determination of Mercury(II) Ion in Real Samples Based upon a New Schiff Base Derivative as an Ionophore
Abstract
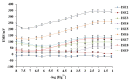
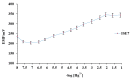
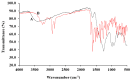
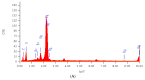
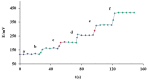
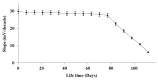
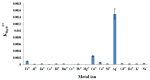
The present paper provides a description of the design, characterization, and use of a Hg2+ selective electrode (Hg2+-SE) for the determination of Hg2+ at ultra-traces levels in a variety of real samples. The ionophore in the proposed electrode is a new Schiff base, namely 4-bromo-2-[(4-methoxyphenylimino)methyl]phenol (BMPMP). All factors affecting electrode response including polymeric membrane composition, concentration of internal solution, pH sample solution, and response time were optimized. The optimum response of our electrode was obtained with the following polymeric membrane composition (% w/w): PVC, 32; o-NPOE, 64.5; BMPMP, 2 and NaTPB, 1.5. The potentiometric response of Hg2+-SE towards Hg2+ ion was linear in the wide range of concentrations (9.33 × 10-8-3.98 × 10-3 molL-1), while, the limit of detection of the proposed electrode was 3.98 × 10-8 molL-1 (8.00 μg L-1). The Hg2+-SE responds quickly to Hg2+ ions as the response time of less than 10 s. On the other hand, the slope value obtained for the developed electrode was 29.74 ± 0.1 mV/decade in the pH range of 2.0-9.0 in good agreement with the Nernstian response (29.50 mV/decade). The Hg2+-SE has relatively less interference with other metal ions. The Hg2+-SE was used as an indicator electrode in potentiometric titrations to estimate Hg2+ ions in waters, compact fluorescent lamp, and dental amalgam alloy and the accuracy of the developed electrode was compared with ICP-OES measurement values. Moreover, the new Schiff base (BMPMP) was synthesized and characterized using ATR-FTIR, elemental analysis, 1H NMR, and 13C NMR. The PVC membranes containing BMPMP as an ionophore unloaded and loaded with Hg(II) are reported by scanning electron microscope images (SEM) along with energy-dispersive X-ray spectroscopy (EDX) spectra.
Keywords: ISE-Hg; PVC membrane; Schiff base; ionophore; mercury selective electrode.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
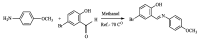





References
-
- Cheng H., Wu C., Liu J., Xu Z. Thiol-functionalized silica microspheres for online preconcentration and determination of mercury species in seawater by high performance liquid chromatography and inductively coupled plasma mass spectrometry. RSC Adv. 2015;5:19082–19090. doi: 10.1039/C4RA13941K. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

