S100P Interacts with p53 while Pentamidine Inhibits This Interaction
- PMID: 33923162
- PMCID: PMC8145327
- DOI: 10.3390/biom11050634
S100P Interacts with p53 while Pentamidine Inhibits This Interaction
Abstract
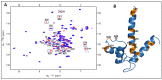
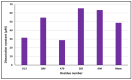
S100P, a small calcium-binding protein, associates with the p53 protein with micromolar affinity. It has been hypothesized that the oncogenic function of S100P may involve binding-induced inactivation of p53. We used 1H-15N HSQC experiments and molecular modeling to study the molecular interactions between S100P and p53 in the presence and absence of pentamidine. Our experimental analysis indicates that the S100P-53 complex formation is successfully disrupted by pentamidine, since S100P shares the same binding site for p53 and pentamidine. In addition, we showed that pentamidine treatment of ZR-75-1 breast cancer cells resulted in reduced proliferation and increased p53 and p21 protein levels, indicating that pentamidine is an effective antagonist that interferes with the S100P-p53 interaction, leading to re-activation of the p53-21 pathway and inhibition of cancer cell proliferation. Collectively, our findings suggest that blocking the association between S100P and p53 by pentamidine will prevent cancer progression and, therefore, provide a new avenue for cancer therapy by targeting the S100P-p53 interaction.
Keywords: 1H-5N HSQC spectrum; HADDOCK program; S100P; biomolecular docking; p53-TAD (73 amino acids); protein-protein interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

