Zinc, Zinc Transporters, and Cadmium Cytotoxicity in a Cell Culture Model of Human Urothelium
- PMID: 33923173
- PMCID: PMC8145463
- DOI: 10.3390/toxics9050094
Zinc, Zinc Transporters, and Cadmium Cytotoxicity in a Cell Culture Model of Human Urothelium
Abstract
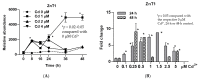
We explored the potential role of zinc (Zn) and zinc transporters in protection against cytotoxicity of cadmium (Cd) in a cell culture model of human urothelium, named UROtsa. We used real-time qRT-PCR to quantify transcript levels of 19 Zn transporters of the Zrt-/Irt-like protein (ZIP) and ZnT gene families that were expressed in UROtsa cells and were altered by Cd exposure. Cd as low as 0.1 µM induced expression of ZnT1, known to mediate efflux of Zn and Cd. Loss of cell viability by 57% was seen 24 h after exposure to 2.5 µM Cd. Exposure to 2.5 µM Cd together with 10-50 µM Zn prevented loss of cell viability by 66%. Pretreatment of the UROtsa cells with an inhibitor of glutathione biosynthesis (buthionine sulfoximine) diminished ZnT1 induction by Cd with a resultant increase in sensitivity to Cd cytotoxicity. Conversely, pretreatment of UROtsa cells with an inhibitor of DNA methylation, 5-aza-2'-deoxycytidine (aza-dC) did not change the extent of ZnT1 induction by Cd. The induced expression of ZnT1 that remained impervious in cells treated with aza-dC coincided with resistance to Cd cytotoxicity. Therefore, expression of ZnT1 efflux transporter and Cd toxicity in UROtsa cells could be modulated, in part, by DNA methylation and glutathione biosynthesis. Induced expression of ZnT1 may be a viable mechanistic approach to mitigating cytotoxicity of Cd.
Keywords: BSO; DNA methylation; ZIP zinc transporters; ZIP14; ZnT zinc transporters; ZnT1; aza-dC; cadmium; glutathione; qRT-PCR; urothelium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Satarug S., Phelps K.R. Cadmium Exposure and Toxicity. In: Bagchi D., Bagchi M., editors. Metal Toxicology Handbook. CRC Press; Boca Raton, FL, USA: 2021. pp. 219–274.
LinkOut - more resources
Full Text Sources

