Glutathione Metabolism and the Novel Role of Mitochondrial GSH in Retinal Degeneration
- PMID: 33923192
- PMCID: PMC8146950
- DOI: 10.3390/antiox10050661
Glutathione Metabolism and the Novel Role of Mitochondrial GSH in Retinal Degeneration
Abstract
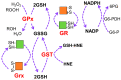
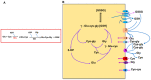
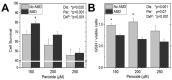
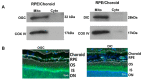
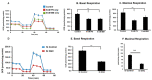
Glutathione (GSH) is present ubiquitously, and its role as a crucial cellular antioxidant in tissues, including the retina, is well established. GSH's antioxidant function arises from its ability to scavenge reactive oxygen species or to serve as an essential cofactor for GSH S-transferases and peroxidases. This review summarizes the general functions, retinal distribution, disorders linked to GSH deficiency, and the emerging role for mitochondrial GSH (mGSH) in retinal function. Though synthesized only in the cytosol, the presence of GSH in multiple cell organelles suggests the requirement for its active transport across organellar membranes. The localization and distribution of 2-oxoglutarate carrier (OGC) and dicarboxylate carrier (DIC), two recently characterized mitochondrial carrier proteins in RPE and retina, show that these transporters are highly expressed in human retinal pigment epithelium (RPE) cells and retinal layers, and their expression increases with RPE polarity in cultured cells. Depletion of mGSH levels via inhibition of the two transporters resulted in reduced mitochondrial bioenergetic parameters (basal respiration, ATP production, maximal respiration, and spare respiratory capacity) and increased RPE cell death. These results begin to reveal a critical role for mGSH in maintaining RPE bioenergetics and cell health. Thus, augmentation of mGSH pool under GSH-deficient conditions may be a valuable tool in treating retinal disorders, such as age-related macular degeneration and optic neuropathies, whose pathologies have been associated with mitochondrial dysfunction.
Keywords: RPE; SLC25A10 (DIC); SLC25A11 (OGC); bioenergetics; mitochondrial GSH; retinal degeneration.
Conflict of interest statement
D.A.F. is a member of the Scientific Advisor Board for Vinci Pharmaceuticals, Inc. R.K. and P.G.S. declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Kwon D.H., Lee H., Park C., Hong S.-H., Hong S.H., Kim G.-Y., Cha H.-J., Kim S., Kim H.-S., Hwang H.-J., et al. Glutathione Induced Immune-Stimulatory Activity by Promoting M1-Like Macrophages Polarization via Potential ROS Scavenging Capacity. Antioxidants. 2019;8:413. doi: 10.3390/antiox8090413. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

