Antivirulence Properties of a Low-Molecular-Weight Quaternized Chitosan Derivative against Pseudomonas aeruginosa
- PMID: 33923269
- PMCID: PMC8145479
- DOI: 10.3390/microorganisms9050912
Antivirulence Properties of a Low-Molecular-Weight Quaternized Chitosan Derivative against Pseudomonas aeruginosa
Abstract
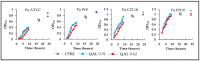
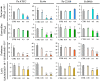
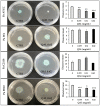
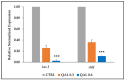
The co-occurrence of increasing rates of resistance to current antibiotics and the paucity of novel antibiotics pose major challenges for the treatment of bacterial infections. In this scenario, treatments targeting bacterial virulence have gained considerable interest as they are expected to exert a weaker selection for resistance than conventional antibiotics. In a previous study, we demonstrated that a low-molecular-weight quaternized chitosan derivative, named QAL, displays antibiofilm activity against the major pathogen Pseudomonas aeruginosa at subinhibitory concentrations. The aim of this study was to investigate whether QAL was able to inhibit the production of relevant virulence factors of P. aeruginosa. When tested in vitro at subinhibiting concentrations (0.31-0.62 mg/mL), QAL markedly reduced the production of pyocyanin, pyoverdin, proteases, and LasA, as well as inhibited the swarming motility of three out of four P. aeruginosa strains tested. Furthermore, quantitative reverse transcription PCR (qRT-PCR) analyses demonstrated that expression of lasI and rhlI, two QS-related genes, was highly downregulated in a representative P. aeruginosa strain. Confocal scanning laser microscopy analysis suggested that FITC-labelled QAL accumulates intracellularly following incubation with P. aeruginosa. In contrast, the reduced production of virulence factors was not evidenced when QAL was used as the main polymeric component of polyelectrolyte-based nanoparticles. Additionally, combination of sub-MIC concentrations of QAL and tobramycin significantly reduced biofilm formation of P. aeruginosa, likely due to a synergistic activity towards planktonic bacteria. Overall, the results obtained demonstrated an antivirulence activity of QAL, possibly due to polymer intracellular localization and QS-inhibition, and its ability to inhibit P. aeruginosa growth synergizing with tobramycin.
Keywords: LasA; Pseudomonas aeruginosa; biofilm; chitosan; motility; proteases; pyocyanin; pyoverdin; quaternized chitosan; quorum sensing; tobramycin; virulence factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Effect of chitosan nanoparticles on quorum sensing-controlled virulence factors and expression of LasI and RhlI genes among Pseudomonas aeruginosa clinical isolates.AIMS Microbiol. 2021 Oct 26;7(4):415-430. doi: 10.3934/microbiol.2021025. eCollection 2021. AIMS Microbiol. 2021. PMID: 35071940 Free PMC article.
-
Musa acuminata and its bioactive metabolite 5-Hydroxymethylfurfural mitigates quorum sensing (las and rhl) mediated biofilm and virulence production of nosocomial pathogen Pseudomonas aeruginosa in vitro.J Ethnopharmacol. 2020 Jan 10;246:112242. doi: 10.1016/j.jep.2019.112242. Epub 2019 Sep 15. J Ethnopharmacol. 2020. PMID: 31533077
-
Antipathogenic Compounds That Are Effective at Very Low Concentrations and Have Both Antibiofilm and Antivirulence Effects against Pseudomonas aeruginosa.Microbiol Spectr. 2021 Oct 31;9(2):e0024921. doi: 10.1128/Spectrum.00249-21. Epub 2021 Sep 8. Microbiol Spectr. 2021. PMID: 34494853 Free PMC article.
-
Chitosan-Aspirin Combination Inhibits Quorum-Sensing Synthases (lasI and rhlI) in Pseudomonas aeruginosa.Life (Basel). 2024 Apr 5;14(4):481. doi: 10.3390/life14040481. Life (Basel). 2024. PMID: 38672752 Free PMC article.
-
Exploration of the inhibitory effect of Cassia fistula on quorum sensing mediated virulence factor production and biofilm activity in Pseudomonas aeruginosa: an in vivo study in model organism Caenorhabditis elegans.J Med Microbiol. 2023 Feb;72(2). doi: 10.1099/jmm.0.001578. J Med Microbiol. 2023. PMID: 36787160
Cited by
-
Quorum quenching effect of cyclodextrins on the pyocyanin and pyoverdine production of Pseudomonas aeruginosa.Appl Microbiol Biotechnol. 2024 Mar 22;108(1):271. doi: 10.1007/s00253-024-13104-7. Appl Microbiol Biotechnol. 2024. PMID: 38517512 Free PMC article.
-
Long-Chain Alkylthio Cyclodextrin Derivatives for Modulation of Quorum-Sensing-Based Bioluminescence in Aliivibrio fischeri Model System.Int J Mol Sci. 2024 Jun 28;25(13):7139. doi: 10.3390/ijms25137139. Int J Mol Sci. 2024. PMID: 39000246 Free PMC article.
-
Exploring the antimicrobial potential of chitosan nanoparticles: synthesis, characterization and impact on Pseudomonas aeruginosa virulence factors.Nanoscale Adv. 2024 Apr 22;6(12):3093-3105. doi: 10.1039/d4na00064a. eCollection 2024 Jun 11. Nanoscale Adv. 2024. PMID: 38868829 Free PMC article.
-
Synthesis and study of antibiofilm and antivirulence properties of flavonol analogues generated by palladium catalyzed ligand free Suzuki-Miyaura coupling against Pseudomonas aeruginosa PAO1.RSC Adv. 2024 Apr 16;14(18):12278-12293. doi: 10.1039/d3ra08617h. eCollection 2024 Apr 16. RSC Adv. 2024. PMID: 38633488 Free PMC article.
-
Chitosan-Based Multifunctional Biomaterials as Active Agents or Delivery Systems for Antibacterial Therapy.Bioengineering (Basel). 2024 Dec 16;11(12):1278. doi: 10.3390/bioengineering11121278. Bioengineering (Basel). 2024. PMID: 39768096 Free PMC article. Review.
References
-
- Raman G., Avendano E.E., Chan J., Merchant S., Puzniak L. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2018;7 doi: 10.1186/s13756-018-0370-9. - DOI - PMC - PubMed
-
- Rello J., Kalwaje Eshwara V., Lagunes L., Alves J., Wunderink R.G., Conway-Morris A., Rojas J.N., Alp E., Zhang Z. A global priority list of the TOp TEn resistant microorganisms (TOTEM) study at intensive care: A prioritization exercise based on multi-criteria decision analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:319–323. doi: 10.1007/s10096-018-3428-y. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

