Tumour Hypoxia-Mediated Immunosuppression: Mechanisms and Therapeutic Approaches to Improve Cancer Immunotherapy
- PMID: 33923305
- PMCID: PMC8146304
- DOI: 10.3390/cells10051006
Tumour Hypoxia-Mediated Immunosuppression: Mechanisms and Therapeutic Approaches to Improve Cancer Immunotherapy
Abstract
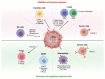
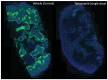
The magnitude of the host immune response can be regulated by either stimulatory or inhibitory immune checkpoint molecules. Receptor-ligand binding between inhibitory molecules is often exploited by tumours to suppress anti-tumour immune responses. Immune checkpoint inhibitors that block these inhibitory interactions can relieve T-cells from negative regulation, and have yielded remarkable activity in the clinic. Despite this success, clinical data reveal that durable responses are limited to a minority of patients and malignancies, indicating the presence of underlying resistance mechanisms. Accumulating evidence suggests that tumour hypoxia, a pervasive feature of many solid cancers, is a critical phenomenon involved in suppressing the anti-tumour immune response generated by checkpoint inhibitors. In this review, we discuss the mechanisms associated with hypoxia-mediate immunosuppression and focus on modulating tumour hypoxia as an approach to improve immunotherapy responsiveness.
Keywords: CP-506; HIF; checkpoint inhibitor; evofosfamide; hypoxia; hypoxia-activated prodrug; immune suppression; immunotherapy; oncolytic virus; tarloxotinib.
Conflict of interest statement
The authors declare authorship of patents relating to tarloxotinib and CP-506 that are licensed or assigned by Auckland Uniservices Limited to third parties for the purpose of commercial development. The funders had no role in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Balar A.V., Galsky M.D., Rosenberg J.E., Powles T., Petrylak D.P., Bellmunt J., Loriot Y., Necchi A., Hoffman-Censits J., Perez-Gracia J.L., et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76. doi: 10.1016/S0140-6736(16)32455-2. - DOI - PMC - PubMed
-
- Patel S.P. Immune checkpoint blockade for lung cancer: State of the art. Transl. Cancer Res. 2015;4:415–422.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

