Effects of Ipriflavone-Loaded Mesoporous Nanospheres on the Differentiation of Endothelial Progenitor Cells and Their Modulation by Macrophages
- PMID: 33923311
- PMCID: PMC8145259
- DOI: 10.3390/nano11051102
Effects of Ipriflavone-Loaded Mesoporous Nanospheres on the Differentiation of Endothelial Progenitor Cells and Their Modulation by Macrophages
Abstract
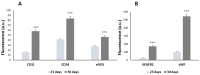
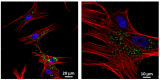
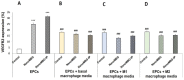
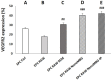
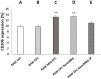
Angiogenic biomaterials are designed to promote vascularization and tissue regeneration. Nanoparticles of bioactive materials loaded with drugs represent an interesting strategy to stimulate osteogenesis and angiogenesis and to inhibit bone resorption. In this work, porcine endothelial progenitor cells (EPCs), essential for blood vessel formation, were isolated and characterized to evaluate the in vitro effects of unloaded (NanoMBGs) and ipriflavone-loaded nanospheres (NanoMBG-IPs), which were designed to prevent osteoporosis. The expression of vascular endothelial growth factor receptor 2 (VEGFR2) was studied in EPCs under different culture conditions: (a) treatment with NanoMBGs or NanoMBG-IPs, (b) culture with media from basal, M1, and M2 macrophages previously treated with NanoMBGs or NanoMBG-IPs, (c) coculture with macrophages in the presence of NanoMBGs or NanoMBG-IPs, and (d) coculture with M2d angiogenic macrophages. The endocytic mechanisms for nanosphere incorporation by EPCs were identified using six different endocytosis inhibitors. The results evidence the great potential of these nanomaterials to enhance VEGFR2 expression and angiogenesis, after intracellular incorporation by EPCs through clathrin-dependent endocytosis, phagocytosis, and caveolae-mediated uptake. The treatment of EPCs with basal, M1, and M2 macrophage culture media and EPC/macrophage coculture studies also confirmed the angiogenic effect of these nanospheres on EPCs, even in the presence of phagocytic cells.
Keywords: endocytosis; endothelial progenitor cells; ipriflavone; macrophages; mesoporous nanospheres; vascular endothelial growth factor receptor 2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Osteoimmune Properties of Mesoporous Bioactive Nanospheres: A Study on T Helper Lymphocytes.Nanomaterials (Basel). 2023 Jul 26;13(15):2183. doi: 10.3390/nano13152183. Nanomaterials (Basel). 2023. PMID: 37570501 Free PMC article.
-
Incorporation and effects of mesoporous SiO2-CaO nanospheres loaded with ipriflavone on osteoblast/osteoclast cocultures.Eur J Pharm Biopharm. 2018 Dec;133:258-268. doi: 10.1016/j.ejpb.2018.10.019. Epub 2018 Oct 29. Eur J Pharm Biopharm. 2018. PMID: 30385420
-
Ipriflavone-Loaded Mesoporous Nanospheres with Potential Applications for Periodontal Treatment.Nanomaterials (Basel). 2020 Dec 21;10(12):2573. doi: 10.3390/nano10122573. Nanomaterials (Basel). 2020. PMID: 33371499 Free PMC article.
-
Endothelial progenitor cells in angiogenesis.Sheng Li Xue Bao. 2005 Feb 25;57(1):1-6. Sheng Li Xue Bao. 2005. PMID: 15719128 Review.
-
Shear stress: An essential driver of endothelial progenitor cells.J Mol Cell Cardiol. 2018 May;118:46-69. doi: 10.1016/j.yjmcc.2018.03.007. Epub 2018 Mar 13. J Mol Cell Cardiol. 2018. PMID: 29549046 Review.
Cited by
-
Crosstalk Between H-Type Vascular Endothelial Cells and Macrophages: A Potential Regulator of Bone Homeostasis.J Inflamm Res. 2025 Feb 25;18:2743-2765. doi: 10.2147/JIR.S502604. eCollection 2025. J Inflamm Res. 2025. PMID: 40026304 Free PMC article. Review.
-
Unravelling the Programmed Inflammation and Tissue Repair by a Multipotential Antimicrobial K21 Silane.Int Dent J. 2025 Apr;75(2):1277-1291. doi: 10.1016/j.identj.2024.09.012. Epub 2024 Sep 24. Int Dent J. 2025. PMID: 39322516 Free PMC article.
-
Mesoporous bioactive glasses for regenerative medicine.Mater Today Bio. 2021 Jun 29;11:100121. doi: 10.1016/j.mtbio.2021.100121. eCollection 2021 Jun. Mater Today Bio. 2021. PMID: 34377972 Free PMC article. Review.
-
Osteoimmune Properties of Mesoporous Bioactive Nanospheres: A Study on T Helper Lymphocytes.Nanomaterials (Basel). 2023 Jul 26;13(15):2183. doi: 10.3390/nano13152183. Nanomaterials (Basel). 2023. PMID: 37570501 Free PMC article.
-
Nidogen1-enriched extracellular vesicles accelerate angiogenesis and bone regeneration by targeting Myosin-10 to regulate endothelial cell adhesion.Bioact Mater. 2021 Oct 27;12:185-197. doi: 10.1016/j.bioactmat.2021.10.021. eCollection 2022 Jun. Bioact Mater. 2021. PMID: 35310379 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

