Neuroprotective Effects of Curcumin in Methamphetamine-Induced Toxicity
- PMID: 33923340
- PMCID: PMC8123176
- DOI: 10.3390/molecules26092493
Neuroprotective Effects of Curcumin in Methamphetamine-Induced Toxicity
Abstract
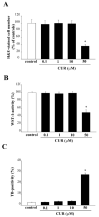
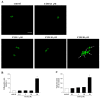
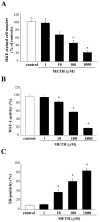
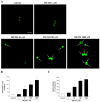
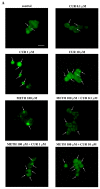
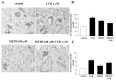
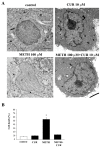
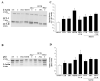
Curcumin (CUR), a natural polyphenol extracted from rhizome of the Curcuma longa L, has received great attention for its multiple potential health benefits as well as disease prevention. For instance, CUR protects against toxic agents acting on the human body, including the nervous system. In detail, CUR possesses, among others, strong effects as an autophagy activator. The present study indicates that CUR counteracts methamphetamine (METH) toxicity. Such a drug of abuse is toxic by disturbing the autophagy machinery. We profited from an unbiased, low variable cell context by using rat pheochromocytoma PC12 cell line. In such a system, a strong protection was exerted by CUR against METH toxicity. This was associated with increased autophagy flux, merging of autophagosomes with lysosomes and replenishment of autophagy vacuoles with LC3, which instead is moved out from the vacuoles by METH. This is expected to enable the autophagy machinery. In fact, while in METH-treated cells the autophagy substrates α-synuclein accumulates in the cytosol, CUR speeds up α-synuclein clearance. Under the effects of CUR LC3 penetrate in autophagy vacuoles to commit them to cell clearance and promotes the autophagy flux. The present data provide evidence that CUR counteracts the neurotoxic effects induced by METH by promoting autophagy.
Keywords: Curcuma longa; LC3; PC12 cells; autophagy; drug of abuse; natural polyphenol; α-synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





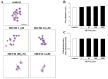
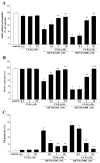
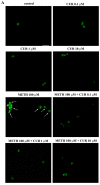






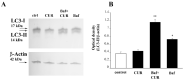




Similar articles
-
Apelin-13 Protects PC12 Cells Against Methamphetamine-Induced Oxidative Stress, Autophagy and Apoptosis.Neurochem Res. 2019 Sep;44(9):2103-2112. doi: 10.1007/s11064-019-02847-9. Epub 2019 Aug 5. Neurochem Res. 2019. PMID: 31385138
-
Norepinephrine Protects against Methamphetamine Toxicity through β2-Adrenergic Receptors Promoting LC3 Compartmentalization.Int J Mol Sci. 2021 Jul 5;22(13):7232. doi: 10.3390/ijms22137232. Int J Mol Sci. 2021. PMID: 34281286 Free PMC article.
-
L-Ascorbate Protects Against Methamphetamine-Induced Neurotoxicity of Cortical Cells via Inhibiting Oxidative Stress, Autophagy, and Apoptosis.Mol Neurobiol. 2017 Jan;54(1):125-136. doi: 10.1007/s12035-015-9561-z. Epub 2016 Jan 5. Mol Neurobiol. 2017. PMID: 26732595
-
Experimental and clinical reports on anti-inflammatory, antioxidant, and immunomodulatory effects of Curcuma longa and curcumin, an updated and comprehensive review.Biofactors. 2021 May;47(3):311-350. doi: 10.1002/biof.1716. Epub 2021 Feb 19. Biofactors. 2021. PMID: 33606322 Review.
-
Potential Role of Curcumin and Its Nanoformulations to Treat Various Types of Cancers.Biomolecules. 2021 Mar 7;11(3):392. doi: 10.3390/biom11030392. Biomolecules. 2021. PMID: 33800000 Free PMC article. Review.
Cited by
-
Bacopa Protects against Neurotoxicity Induced by MPP+ and Methamphetamine.Molecules. 2022 Aug 15;27(16):5204. doi: 10.3390/molecules27165204. Molecules. 2022. PMID: 36014442 Free PMC article.
-
Neuroprotective Effect of Curcumin-Loaded RGD Peptide-PEGylated Nanoliposomes.Pharmaceutics. 2023 Nov 24;15(12):2665. doi: 10.3390/pharmaceutics15122665. Pharmaceutics. 2023. PMID: 38140006 Free PMC article.
-
Molecular mechanisms of programmed cell death in methamphetamine-induced neuronal damage.Front Pharmacol. 2022 Aug 17;13:980340. doi: 10.3389/fphar.2022.980340. eCollection 2022. Front Pharmacol. 2022. PMID: 36059947 Free PMC article.
-
A Scientometric Visualization Analysis for Molecular Mechanisms of Substance Abuse and Its Neurotoxicity From 1997 to 2021.Front Mol Neurosci. 2022 Jul 1;15:885701. doi: 10.3389/fnmol.2022.885701. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35845612 Free PMC article.
-
Autophagy Activation Promoted by Pulses of Light and Phytochemicals Counteracting Oxidative Stress during Age-Related Macular Degeneration.Antioxidants (Basel). 2023 May 30;12(6):1183. doi: 10.3390/antiox12061183. Antioxidants (Basel). 2023. PMID: 37371913 Free PMC article. Review.
References
-
- Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. - DOI - PMC - PubMed
-
- Menon V.P., Sudheer A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007;595:105–125. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

