Rho Family GTPases and Rho GEFs in Glucose Homeostasis
- PMID: 33923452
- PMCID: PMC8074089
- DOI: 10.3390/cells10040915
Rho Family GTPases and Rho GEFs in Glucose Homeostasis
Abstract
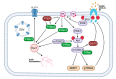
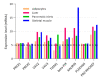
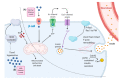
Dysregulation of glucose homeostasis leading to metabolic syndrome and type 2 diabetes is the cause of an increasing world health crisis. New intriguing roles have emerged for Rho family GTPases and their Rho guanine nucleotide exchange factor (GEF) activators in the regulation of glucose homeostasis. This review summates the current knowledge, focusing in particular on the roles of Rho GEFs in the processes of glucose-stimulated insulin secretion by pancreatic β cells and insulin-stimulated glucose uptake into skeletal muscle and adipose tissues. We discuss the ten Rho GEFs that are known so far to regulate glucose homeostasis, nine of which are in mammals, and one is in yeast. Among the mammalian Rho GEFs, P-Rex1, Vav2, Vav3, Tiam1, Kalirin and Plekhg4 were shown to mediate the insulin-stimulated translocation of the glucose transporter GLUT4 to the plasma membrane and/or insulin-stimulated glucose uptake in skeletal muscle or adipose tissue. The Rho GEFs P-Rex1, Vav2, Tiam1 and β-PIX were found to control the glucose-stimulated release of insulin by pancreatic β cells. In vivo studies demonstrated the involvement of the Rho GEFs P-Rex2, Vav2, Vav3 and PDZ-RhoGEF in glucose tolerance and/or insulin sensitivity, with deletion of these GEFs either contributing to the development of metabolic syndrome or protecting from it. This research is in its infancy. Considering that over 80 Rho GEFs exist, it is likely that future research will identify more roles for Rho GEFs in glucose homeostasis.
Keywords: GLUT4 glucose transporter; Rho GEF; Rho GTPase; glucose homeostasis; glucose-stimulated insulin secretion; guanine nucleotide exchange factor; insulin-stimulated glucose uptake; metabolic syndrome; small G protein; type 2 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

