Anti-PD-1/PD-L1 Based Combination Immunotherapy to Boost Antigen-Specific CD8+ T Cell Response in Hepatocellular Carcinoma
- PMID: 33923463
- PMCID: PMC8073815
- DOI: 10.3390/cancers13081922
Anti-PD-1/PD-L1 Based Combination Immunotherapy to Boost Antigen-Specific CD8+ T Cell Response in Hepatocellular Carcinoma
Abstract
Thirty to fifty percent of hepatocellular carcinomas (HCC) display an immune class genetic signature. In this type of tumor, HCC-specific CD8 T cells carry out a key role in HCC control. Those potential reactive HCC-specific CD8 T cells recognize either HCC immunogenic neoantigens or aberrantly expressed host's antigens, but they become progressively exhausted or deleted. These cells express the negative immunoregulatory checkpoint programmed cell death protein 1 (PD-1) which impairs T cell receptor signaling by blocking the CD28 positive co-stimulatory signal. The pool of CD8 cells sensitive to anti-PD-1/PD-L1 treatment is the PD-1dim memory-like precursor pool that gives rise to the effector subset involved in HCC control. Due to the epigenetic imprints that are transmitted to the next generation, the effect of PD-1 blockade is transient, and repeated treatments lead to tumor resistance. During long-lasting disease, besides the TCR signaling impairment, T cells develop other failures that should be also set-up to increase T cell reactivity. Therefore, several PD-1 blockade-based combinatory therapies are currently under investigation such as adding antiangiogenics, anti-TGFβ1, blockade of other negative immune checkpoints, or increasing HCC antigen presentation. The effect of these combinations on CD8+ T cells is discussed in this review.
Keywords: CD8 T cell response; PD-1; PD-L1; combination therapy; hepatocellular carcinoma; immune check-point inhibitor; immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

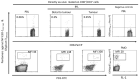
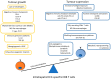
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

