Catalase Modulates the Radio-Sensitization of Pancreatic Cancer Cells by Pharmacological Ascorbate
- PMID: 33923601
- PMCID: PMC8073689
- DOI: 10.3390/antiox10040614
Catalase Modulates the Radio-Sensitization of Pancreatic Cancer Cells by Pharmacological Ascorbate
Abstract
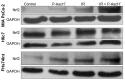
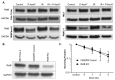
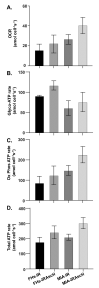
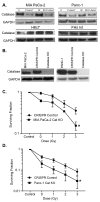
Pancreatic cancer cells (PDACs) are more susceptible to an oxidative insult than normal cells, resulting in greater cytotoxicity upon exposure to agents that increase pro-oxidant levels. Pharmacological ascorbate (P-AscH-), i.e., large amounts given intravenously (IV), generates significant fluxes of hydrogen peroxide (H2O2), resulting in the killing of PDACs but not normal cells. Recent studies have demonstrated that P-AscH- radio-sensitizes PDAC but is a radioprotector to normal cells and tissues. Several mechanisms have been hypothesized to explain the dual roles of P-AscH- in radiation-induced toxicity including the activation of nuclear factor-erythroid 2-related factor 2 (Nrf2), RelB, as well as changes in bioenergetic profiles. We have found that P-AscH- in conjunction with radiation increases Nrf2 in both cancer cells and normal cells. Although P-AscH- with radiation decreases RelB in cancer cells vs. normal cells, the knockout of RelB does not radio-sensitize PDACs. Cellular bioenergetic profiles demonstrate that P-AscH- with radiation increases the ATP demand/production rate (glycolytic and oxidative phosphorylation) in both PDACs and normal cells. Knocking out catalase results in P-AscH- radio-sensitization in PDACs. In a phase I trial where P-AscH- was included as an adjuvant to the standard of care, short-term survivors had higher catalase levels in tumor tissue, compared to long-term survivors. These data suggest that P-AscH- radio-sensitizes PDACs through increased peroxide flux. Catalase levels could be a possible indicator for how tumors will respond to P-AscH-.
Keywords: DNA damage; DNA repair; pancreatic cancer; pharmacological ascorbate; vitamin C.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Auranofin and Pharmacologic Ascorbate as Radiomodulators in the Treatment of Pancreatic Cancer.Antioxidants (Basel). 2022 May 14;11(5):971. doi: 10.3390/antiox11050971. Antioxidants (Basel). 2022. PMID: 35624835 Free PMC article. Review.
-
Pharmacological ascorbate combined with rucosopasem selectively radio-chemo-sensitizes NSCLC via generation of H2O2.Redox Biol. 2025 Mar;80:103505. doi: 10.1016/j.redox.2025.103505. Epub 2025 Jan 23. Redox Biol. 2025. PMID: 39884000 Free PMC article.
-
Hydrogen Peroxide Mediates Pharmacological Ascorbate Induced Radio-Sensitization of Pancreatic Cancer Cells by Enhancing G2-accumulation and Reducing Cyclin B1 Protein Levels.Radiat Res. 2023 Nov 1;200(5):444-455. doi: 10.1667/RADE-22-00182.1. Radiat Res. 2023. PMID: 37758045 Free PMC article.
-
Manipulation of Redox Metabolism Using Pharmacologic Ascorbate Opens a Therapeutic Window for Radio-Sensitization by ATM Inhibitors in Colorectal Cancer.Int J Radiat Oncol Biol Phys. 2023 Mar 15;115(4):933-944. doi: 10.1016/j.ijrobp.2022.10.012. Epub 2022 Oct 11. Int J Radiat Oncol Biol Phys. 2023. PMID: 36228747 Free PMC article.
-
Pharmacological Ascorbate as a Means of Sensitizing Cancer Cells to Radio-Chemotherapy While Protecting Normal Tissue.Semin Radiat Oncol. 2019 Jan;29(1):25-32. doi: 10.1016/j.semradonc.2018.10.006. Semin Radiat Oncol. 2019. PMID: 30573181 Free PMC article. Review.
Cited by
-
Auranofin and Pharmacologic Ascorbate as Radiomodulators in the Treatment of Pancreatic Cancer.Antioxidants (Basel). 2022 May 14;11(5):971. doi: 10.3390/antiox11050971. Antioxidants (Basel). 2022. PMID: 35624835 Free PMC article. Review.
-
Catalase-Knockout Complements the Radio-Sensitization Effect of Titanium Peroxide Nanoparticles on Pancreatic Cancer Cells.Molecules. 2025 Jan 31;30(3):629. doi: 10.3390/molecules30030629. Molecules. 2025. PMID: 39942733 Free PMC article.
-
Targeting catalase in cancer.Redox Biol. 2024 Nov;77:103404. doi: 10.1016/j.redox.2024.103404. Epub 2024 Oct 19. Redox Biol. 2024. PMID: 39447253 Free PMC article. Review.
-
Pharmacologic Ascorbate Radiosensitizes Pancreatic Cancer but Radioprotects Normal Tissue: The Role of Oxidative Stress-Induced Lipid Peroxidation.Antioxidants (Basel). 2024 Mar 18;13(3):361. doi: 10.3390/antiox13030361. Antioxidants (Basel). 2024. PMID: 38539894 Free PMC article.
References
-
- Iacobuzio-Donahue C.A., Fu B., Yachida S., Luo M., Abe H., Henderson C.M., Vilardell F., Wang Z., Keller J.W., Banerjee P., et al. DPC4 Gene Status of the Primary Carcinoma Correlates with Patterns of Failure in Patients with Pancreatic Cancer. J. Clin. Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. - DOI - PMC - PubMed
-
- Oberley L.W., Buettner G.R. Role of Superoxide Dismutase in Cancer: A Review. Cancer Res. 1979;39:1141–1149. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

