Molecular, Immunomodulatory, and Histopathological Role of Mesenchymal Stem Cells and Beetroot Extract on Cisplatin Induced Testicular Damage in Albino Rats
- PMID: 33923635
- PMCID: PMC8074130
- DOI: 10.3390/ani11041142
Molecular, Immunomodulatory, and Histopathological Role of Mesenchymal Stem Cells and Beetroot Extract on Cisplatin Induced Testicular Damage in Albino Rats
Abstract
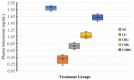
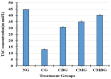
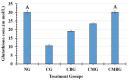
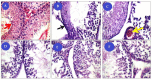
Cisplatin (Cis) a drug commonly used as a chemotherapeutic agent to treat various types of cancer, inducing testicular damage. The present study aimed to investigate the inhibitory potential of bone marrow-derived mesenchymal stem cells (BM-MSCs) and beetroot extract (BRE) in albino rats after testicular toxicity induced by cisplatin. Thirty adult male albino rats were grouped into: the control group, Cis group receiving a single dose of 7 mg/kg i.p. (intraperitoneal) to induce testicular toxicity, Cis plus BM-MSCs injected Cis followed by 2 × 106 of BM-MSCs; Cis plus BRE group receiving Cis followed by 300 mg/kg body weight/day of BRE, and Cis plus BM-MSCs and BRE group. In the current study, Cis reduced sperm count, serum testosterone level, and testicular activity of alkaline phosphatase (AKP), besides a marked inhibition of succinate dehydrogenase (SDH) activity. In addition, it significantly increased malondialdehyde (MDA) and along with a marked decrease in testis reduced glutathione content and total antioxidant capacity (TAC). At the same time, Cis administration resulted in a marked elevation in interleukine-6 and the iNOS and caspase-3 genes; however, it decreased the expression of steroidogenic acute regulatory protein (StAR). Combined treatment with BM-MSCs and BRE resulted in great improvement of all previous parameters. These results were also confirmed by histopathological and immunohistochemical examination. In conclusion, both MSCs and BRE were found to have potent potentials to inhibit testicular damage induced by cisplatin.
Keywords: StAR; beetroot extract; caspase-3; cisplatin; iNOS; mesenchymal stem cells; testicular damage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
The role of mesenchymal stem cells in chemotherapy-induced gonadotoxicity.Stem Cell Res Ther. 2018 Jul 18;9(1):196. doi: 10.1186/s13287-018-0946-6. Stem Cell Res Ther. 2018. PMID: 30021657 Free PMC article.
-
Amelioration of cadmium-induced testes' damage in rats by the bone marrow mesenchymal stem cells.Ecotoxicol Environ Saf. 2018 Feb;148:763-769. doi: 10.1016/j.ecoenv.2017.10.016. Epub 2017 Dec 1. Ecotoxicol Environ Saf. 2018. PMID: 29182986
-
Bone-Marrow-Derived Mesenchymal Stem Cells, Their Conditioned Media, and Olive Leaf Extract Protect against Cisplatin-Induced Toxicity by Alleviating Oxidative Stress, Inflammation, and Apoptosis in Rats.Toxics. 2022 Sep 6;10(9):526. doi: 10.3390/toxics10090526. Toxics. 2022. PMID: 36136492 Free PMC article.
-
The impact of mesenchymal stem cells on doxorubicin-induced testicular toxicity and progeny outcome of male prepubertal rats.Birth Defects Res. 2019 Aug 1;111(13):906-919. doi: 10.1002/bdr2.1535. Epub 2019 Jun 18. Birth Defects Res. 2019. PMID: 31210400
-
Combined effect of bone marrow derived mesenchymal stem cells and nitric oxide inducer on injured gastric mucosa in a rat model.Tissue Cell. 2016 Dec;48(6):644-652. doi: 10.1016/j.tice.2016.09.006. Epub 2016 Sep 28. Tissue Cell. 2016. PMID: 27751517 Review.
Cited by
-
Prevention of Testicular Damage by Indole Derivative MMINA via Upregulated StAR and CatSper Channels with Coincident Suppression of Oxidative Stress and Inflammation: In Silico and In Vivo Validation.Antioxidants (Basel). 2022 Oct 19;11(10):2063. doi: 10.3390/antiox11102063. Antioxidants (Basel). 2022. PMID: 36290786 Free PMC article.
-
Anti-Inflammatory, Anti-Apoptotic, and Antioxidant Roles of Honey, Royal Jelly, and Propolis in Suppressing Nephrotoxicity Induced by Doxorubicin in Male Albino Rats.Antioxidants (Basel). 2022 May 23;11(5):1029. doi: 10.3390/antiox11051029. Antioxidants (Basel). 2022. PMID: 35624893 Free PMC article.
-
Mitigative Effects of l-Arginine and N-Acetyl Cysteine against Cisplatin-Induced Testicular Dysfunction and Toxicity through the Regulation of Antioxidant, Anti-inflammatory, and Antiapoptotic Markers: Role of miR-155 and miR-34c Expression.ACS Omega. 2024 Jun 11;9(25):27680-27691. doi: 10.1021/acsomega.4c03742. eCollection 2024 Jun 25. ACS Omega. 2024. PMID: 38947789 Free PMC article.
-
Ameliorative Effect of Citrus Lemon Peel Extract and Resveratrol on Premature Ovarian Failure Rat Model: Role of iNOS/Caspase-3 Pathway.Molecules. 2022 Dec 23;28(1):122. doi: 10.3390/molecules28010122. Molecules. 2022. PMID: 36615313 Free PMC article.
-
The Combined Effect of Licorice Extract and Bone Marrow Mesenchymal Stem Cells on Cisplatin-Induced Hepatocellular Damage in Rats.Metabolites. 2023 Jan 6;13(1):94. doi: 10.3390/metabo13010094. Metabolites. 2023. PMID: 36677019 Free PMC article.
References
-
- Fallahzadeh A.R., Rezaei Z., Rahimi H.R., Barmak M.J., Sadeghi H., Mehrabi S., Rabani S.M., Kashani I.R., Barati V., Mahmoudi R. Evaluation of the Effect of Pentoxifylline on Cisplatin-Induced Testicular Toxicity in Rats. Toxicol. Res. 2017;33:255–263. doi: 10.5487/TR.2017.33.3.255. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

