Cardiac cAMP-PKA Signaling Compartmentalization in Myocardial Infarction
- PMID: 33923648
- PMCID: PMC8073060
- DOI: 10.3390/cells10040922
Cardiac cAMP-PKA Signaling Compartmentalization in Myocardial Infarction
Abstract
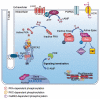
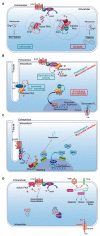
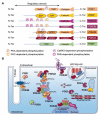
Under physiological conditions, cAMP signaling plays a key role in the regulation of cardiac function. Activation of this intracellular signaling pathway mirrors cardiomyocyte adaptation to various extracellular stimuli. Extracellular ligand binding to seven-transmembrane receptors (also known as GPCRs) with G proteins and adenylyl cyclases (ACs) modulate the intracellular cAMP content. Subsequently, this second messenger triggers activation of specific intracellular downstream effectors that ensure a proper cellular response. Therefore, it is essential for the cell to keep the cAMP signaling highly regulated in space and time. The temporal regulation depends on the activity of ACs and phosphodiesterases. By scaffolding key components of the cAMP signaling machinery, A-kinase anchoring proteins (AKAPs) coordinate both the spatial and temporal regulation. Myocardial infarction is one of the major causes of death in industrialized countries and is characterized by a prolonged cardiac ischemia. This leads to irreversible cardiomyocyte death and impairs cardiac function. Regardless of its causes, a chronic activation of cardiac cAMP signaling is established to compensate this loss. While this adaptation is primarily beneficial for contractile function, it turns out, in the long run, to be deleterious. This review compiles current knowledge about cardiac cAMP compartmentalization under physiological conditions and post-myocardial infarction when it appears to be profoundly impaired.
Keywords: A-kinase anchoring protein; cAMP signaling; cardiomyocytes; heart; myocardial infarction; phosphodiesterases; protein kinase A.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

