Advances in Fabricating the Electrospun Biopolymer-Based Biomaterials
- PMID: 33923664
- PMCID: PMC8167588
- DOI: 10.3390/jfb12020026
Advances in Fabricating the Electrospun Biopolymer-Based Biomaterials
Abstract
Biopolymers formed into a fibrous morphology through electrospinning are of increasing interest in the field of biomedicine due to their intrinsic biocompatibility and biodegradability and their ability to be biomimetic to various fibrous structures present in animal tissues. However, their mechanical properties are often unsatisfactory and their processing may be troublesome. Thus, extensive research interest is focused on improving these qualities. This review article presents the selection of the recent advances in techniques aimed to improve the electrospinnability of various biopolymers (polysaccharides, polynucleotides, peptides, and phospholipids). The electrospinning of single materials, and the variety of co-polymers, with and without additives, is covered. Additionally, various crosslinking strategies are presented. Examples of cytocompatibility, biocompatibility, and antimicrobial properties are analyzed. Special attention is given to whey protein isolate as an example of a novel, promising, green material with good potential in the field of biomedicine. This review ends with a brief summary and outlook for the biomedical applicability of electrospinnable biopolymers.
Keywords: biopolymers; crosslinking strategies; electrospinning; peptides; tissue engineering.
Conflict of interest statement
There are no conflicts of interest to declare. All authors have approved the final version of the manuscript.
Figures



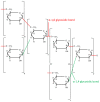
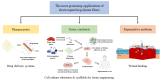
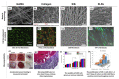
References
-
- Schiffman J.D., Schauer C.L. A Review: Electrospinning of Biopolymer Nanofibers and their Applications. Polym. Rev. 2008;48:317–352. doi: 10.1080/15583720802022182. - DOI
-
- Buschle-Diller G., Hawkins A., Cooper J. Electrospun Nanofibers from Biopolymers and Their Biomedical Applications. In: Edwards J.V., Buschle-Diller G., Goheen S.C., editors. Modified Fibers with Medical and Specialty Applications. Springer; Dordrecht, The Netherlands: 2006. pp. 67–80.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

