Mosaic Segmental and Whole-Chromosome Upd(11)mat in Silver-Russell Syndrome
- PMID: 33923683
- PMCID: PMC8073375
- DOI: 10.3390/genes12040581
Mosaic Segmental and Whole-Chromosome Upd(11)mat in Silver-Russell Syndrome
Abstract
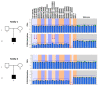
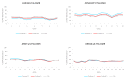
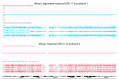
Molecular defects altering the expression of the imprinted genes of the 11p15.5 cluster are responsible for the etiology of two congenital disorders characterized by opposite growth disturbances, Silver-Russell syndrome (SRS), associated with growth restriction, and Beckwith-Wiedemann syndrome (BWS), associated with overgrowth. At the molecular level, SRS and BWS are characterized by defects of opposite sign, including loss (LoM) or gain (GoM) of methylation at the H19/IGF2:intergenic differentially methylated region (H19/IGF2:IG-DMR), maternal or paternal duplication (dup) of 11p15.5, maternal (mat) or paternal (pat) uniparental disomy (upd), and gain or loss of function mutations of CDKN1C. However, while upd(11)pat is found in 20% of BWS cases and in the majority of them it is segmental, upd(11)mat is extremely rare, being reported in only two SRS cases to date, and in both of them is extended to the whole chromosome. Here, we report on two novel cases of mosaic upd(11)mat with SRS phenotype. The upd is mosaic and isodisomic in both cases but covers the entire chromosome in one case and is restricted to 11p14.1-pter in the other case. The segmental upd(11)mat adds further to the list of molecular defects of opposite sign in SRS and BWS, making these two imprinting disorders even more specular than previously described.
Keywords: Beckwith–Wiedemann syndrome; Silver–Russell syndrome; genomic imprinting; imprinting disorders; uniparental disomy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Azzi S., Salem J., Thibaud N., Chantot-Bastaraud S., Lieber E., Netchine I., Harbison M.D. A prospective study validating a clinical scoring system and demonstrating phenotypical-genotypical correlations in Silver-Russell syndrome. J. Med. Genet. 2015;52:446–453. doi: 10.1136/jmedgenet-2014-102979. - DOI - PMC - PubMed
-
- Wakeling E.L., Brioude F., Lokulo-Sodipe O., O’Connell S.M., Salem J., Bliek J., Canton A.P., Chrzanowska K.H., Davies J.H., Dias R.P., et al. Diagnosis and management of Silver-Russell syndrome: First international consensus statement. Nat. Rev. Endocrinol. 2017;13:105–124. doi: 10.1038/nrendo.2016.138. - DOI - PubMed
-
- Soellner L., Kraft F., Sauer S., Begemann M., Kurth I., Elbracht M., Eggermann T. Search for cis-acting factors and maternal effect variants in Silver-Russell patients with ICR1 hypomethylation and their mothers. Eur. J. Hum. Genet. 2019;27:42–48. doi: 10.1038/s41431-018-0269-1. - DOI - PMC - PubMed
-
- Azzi S., Rossignol S., Steunou V., Sas T., Thibaud N., Danton F., Le Jule M., Heinrichs C., Cabrol S., Gicquel C., et al. Multilocus methylation analysis in a large cohort of 11p15-related foetal growth disorders (Russell Silver and Beckwith Wiedemann syndromes) reveals simultaneous loss of methylation at paternal and maternal imprinted loci. Hum. Mol. Genet. 2009;18:4724–4733. doi: 10.1093/hmg/ddp435. - DOI - PubMed
-
- Brioude F., Oliver-Petit I., Blaise A., Praz F., Rossignol S., Le Jule M., Thibaud N., Faussat A.M., Tauber M., Le Bouc Y., et al. CDKN1C mutation affecting the PCNA-binding domain as a cause of familial Russell Silver syndrome. J. Med. Genet. 2013;50:823–830. doi: 10.1136/jmedgenet-2013-101691. - DOI - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

