Maternal Heat Stress Alters Expression of Genes Associated with Nutrient Transport Activity and Metabolism in Female Placentae from Mid-Gestating Pigs
- PMID: 33923747
- PMCID: PMC8073098
- DOI: 10.3390/ijms22084147
Maternal Heat Stress Alters Expression of Genes Associated with Nutrient Transport Activity and Metabolism in Female Placentae from Mid-Gestating Pigs
Abstract
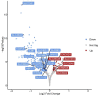
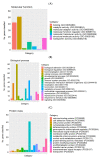
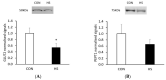
Placental insufficiency is a known consequence of maternal heat stress during gestation in farm animals. The molecular regulation of placentae during the stress response is little known in pigs. This study aims to identify differential gene expression in pig placentae caused by maternal heat exposure during early to mid-gestation. RNA sequencing (RNA-seq) was performed on female placental samples from pregnant pigs exposed to thermoneutral control (CON; constant 20 °C; n = 5) or cyclic heat stress (HS; cyclic 28 to 33 °C; n = 5) conditions between d40 and d60 of gestation. On d60 of gestation, placental efficiency (fetal/placental weight) was decreased (p = 0.023) by maternal HS. A total of 169 genes were differentially expressed (FDR ≤ 0.1) between CON and HS placentae of female fetuses, of which 35 genes were upregulated and 134 genes were downregulated by maternal HS. The current data revealed transport activity (FDR = 0.027), glycoprotein biosynthetic process (FDR = 0.044), and carbohydrate metabolic process (FDR = 0.049) among the terms enriched by the downregulated genes (HS vs. CON). In addition, solute carrier (SLC)-mediated transmembrane transport (FDR = 0.008) and glycosaminoglycan biosynthesis (FDR = 0.027), which modulates placental stroma synthesis, were identified among the pathways enriched by the downregulated genes. These findings provide evidence that heat-stress induced placental inefficiency may be underpinned by altered expression of genes associated with placental nutrient transport capacity and metabolism. A further understanding of the molecular mechanism contributes to the identification of placental gene signatures of summer infertility in pigs.
Keywords: RNA-seq; gestation; heat stress; pigs; placenta; placental insufficiency; transport activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bell A., Wilkening R., Meschia G. Some aspects of placental function in chronically heat-stressed ewes. J. Dev. Physiol. 1987;9:17–29. - PubMed
-
- Bell A. Prenatal programming of postnatal productivity and health of livestock: A brief review. Aust. J. Exp. Agric. 2006;46:725–732. doi: 10.1071/EA06006. - DOI
-
- Liu F., Ford E.M., Morrison R.S., Brewster C.J., Henman D.J., Smits R.J., Zhao W., Cottrell J.J., Leury B.J., Dunshea F.R. The Greater Proportion of Born-Light Progeny from Sows Mated in Summer Contributes to Increased Carcass Fatness Observed in Spring. Animals. 2020;10:2080. doi: 10.3390/ani10112080. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

