Insights into the Pathogenesis of Viral Haemorrhagic Fever Based on Virus Tropism and Tissue Lesions of Natural Rift Valley Fever
- PMID: 33923863
- PMCID: PMC8073615
- DOI: 10.3390/v13040709
Insights into the Pathogenesis of Viral Haemorrhagic Fever Based on Virus Tropism and Tissue Lesions of Natural Rift Valley Fever
Abstract
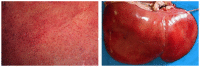
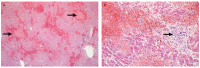
Rift Valley fever phlebovirus (RVFV) infects humans and a wide range of ungulates and historically has caused devastating epidemics in Africa and the Arabian Peninsula. Lesions of naturally infected cases of Rift Valley fever (RVF) have only been described in detail in sheep with a few reports concerning cattle and humans. The most frequently observed lesion in both ruminants and humans is randomly distributed necrosis, particularly in the liver. Lesions supportive of vascular endothelial injury are also present and include mild hydropericardium, hydrothorax and ascites; marked pulmonary congestion and oedema; lymph node congestion and oedema; and haemorrhages in many tissues. Although a complete understanding of RVF pathogenesis is still lacking, antigen-presenting cells in the skin are likely the early targets of the virus. Following suppression of type I IFN production and necrosis of dermal cells, RVFV spreads systemically, resulting in infection and necrosis of other cells in a variety of organs. Failure of both the innate and adaptive immune responses to control infection is exacerbated by apoptosis of lymphocytes. An excessive pro-inflammatory cytokine and chemokine response leads to microcirculatory dysfunction. Additionally, impairment of the coagulation system results in widespread haemorrhages. Fatal outcomes result from multiorgan failure, oedema in many organs (including the lungs and brain), hypotension, and circulatory shock. Here, we summarize current understanding of RVF cellular tropism as informed by lesions caused by natural infections. We specifically examine how extant knowledge informs current understanding regarding pathogenesis of the haemorrhagic fever form of RVF, identifying opportunities for future research.
Keywords: Bunyavirales; Rift Valley fever phlebovirus; arbovirus; emerging diseases; pathogenesis; pathology; tropism; zoonotic disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






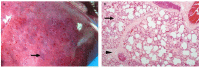


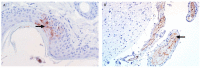


Similar articles
-
Lesions and Cellular Tropism of Natural Rift Valley Fever Virus Infection in Adult Sheep.Vet Pathol. 2019 Jan;56(1):61-77. doi: 10.1177/0300985818806049. Epub 2018 Oct 21. Vet Pathol. 2019. PMID: 30343650
-
Lesions and Cellular Tropism of Natural Rift Valley Fever Virus Infection in Young Lambs.Vet Pathol. 2020 Jan;57(1):66-81. doi: 10.1177/0300985819882633. Epub 2019 Dec 17. Vet Pathol. 2020. PMID: 31842723
-
Ovine Fetal and Placental Lesions and Cellular Tropism in Natural Rift Valley Fever Virus Infections.Vet Pathol. 2020 Nov;57(6):791-806. doi: 10.1177/0300985820954549. Epub 2020 Sep 4. Vet Pathol. 2020. PMID: 32885745
-
The pathogenesis of Rift Valley fever.Viruses. 2011 May;3(5):493-519. doi: 10.3390/v3050493. Viruses. 2011. PMID: 21666766 Free PMC article. Review.
-
Rift Valley Fever.Vet Clin North Am Food Anim Pract. 2024 Jul;40(2):293-304. doi: 10.1016/j.cvfa.2024.01.004. Epub 2024 Mar 6. Vet Clin North Am Food Anim Pract. 2024. PMID: 38453549 Review.
Cited by
-
Anti-Schmallenberg Virus Activities of Type I/III Interferons-Induced Mx1 GTPases from Different Mammalian Species.Viruses. 2023 Apr 25;15(5):1055. doi: 10.3390/v15051055. Viruses. 2023. PMID: 37243140 Free PMC article.
-
The public health threat of emerging phenuiviruses.One Health. 2025 May 6;20:101055. doi: 10.1016/j.onehlt.2025.101055. eCollection 2025 Jun. One Health. 2025. PMID: 40630111 Free PMC article. Review.
-
Intact Type I Interferon Receptor Signaling Prevents Hepatocellular Necrosis but Not Encephalitis in a Dose-Dependent Manner in Rift Valley Fever Virus Infected Mice.Int J Mol Sci. 2022 Oct 18;23(20):12492. doi: 10.3390/ijms232012492. Int J Mol Sci. 2022. PMID: 36293352 Free PMC article.
-
How Viruses Use the VCP/p97 ATPase Molecular Machine.Viruses. 2021 Sep 21;13(9):1881. doi: 10.3390/v13091881. Viruses. 2021. PMID: 34578461 Free PMC article. Review.
-
Emerging and reemerging infectious diseases: global trends and new strategies for their prevention and control.Signal Transduct Target Ther. 2024 Sep 11;9(1):223. doi: 10.1038/s41392-024-01917-x. Signal Transduct Target Ther. 2024. PMID: 39256346 Free PMC article. Review.
References
-
- Anyangu A.S., Gould L.H., Sharif S.K., Nguku P.M., Omolo J.O., Mutonga D., Rao C.Y., Lederman E.R., Schnabel D., Paweska J.T., et al. Risk factors for severe Rift Valley fever infection in Kenya, 2007. Am. J. Trop. Med. Hyg. 2010;83(Suppl. 2):14–21. doi: 10.4269/ajtmh.2010.09-0293. - DOI - PMC - PubMed
-
- Madani T.A., Al-Mazrou Y.Y., Al-Jeffri M.H., Mishkhas A.A., Al-Rabeah A.M., Turkistani A.M., Al-Sayed M.O., Abodahish A.A., Khan A.S., Ksiazek T.G., et al. Rift Valley fever epidemic in Saudi Arabia: Epidemiological, clinical, and laboratory characteristics. Clin. Infect. Dis. 2003;37:1084–1092. doi: 10.1086/378747. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

