Role of Proteasomes in Inflammation
- PMID: 33923887
- PMCID: PMC8072576
- DOI: 10.3390/jcm10081783
Role of Proteasomes in Inflammation
Abstract
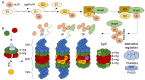
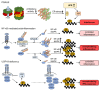
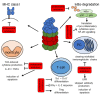
The ubiquitin-proteasome system (UPS) is involved in multiple cellular functions including the regulation of protein homeostasis, major histocompatibility (MHC) class I antigen processing, cell cycle proliferation and signaling. In humans, proteasome loss-of-function mutations result in autoinflammation dominated by a prominent type I interferon (IFN) gene signature. These genomic alterations typically cause the development of proteasome-associated autoinflammatory syndromes (PRAAS) by impairing proteasome activity and perturbing protein homeostasis. However, an abnormal increased proteasomal activity can also be found in other human inflammatory diseases. In this review, we cast a light on the different clinical aspects of proteasomal activity in human disease and summarize the currently studied therapeutic approaches.
Keywords: autoimmune; autoinflammation; inflammation; proteasome; proteasome-associated autoinflammatory syndrome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Contribution of the Unfolded Protein Response (UPR) to the Pathogenesis of Proteasome-Associated Autoinflammatory Syndromes (PRAAS).Front Immunol. 2019 Nov 26;10:2756. doi: 10.3389/fimmu.2019.02756. eCollection 2019. Front Immunol. 2019. PMID: 31827472 Free PMC article. Review.
-
Hematopoietic stem cell transplantation in a patient with proteasome-associated autoinflammatory syndrome (PRAAS).J Allergy Clin Immunol. 2022 Mar;149(3):1120-1127.e8. doi: 10.1016/j.jaci.2021.07.039. Epub 2021 Aug 17. J Allergy Clin Immunol. 2022. PMID: 34416217
-
Identification of eight novel proteasome variants in five unrelated cases of proteasome-associated autoinflammatory syndromes (PRAAS).Front Immunol. 2023 Aug 4;14:1190104. doi: 10.3389/fimmu.2023.1190104. eCollection 2023. Front Immunol. 2023. PMID: 37600812 Free PMC article.
-
Dysregulation of immunoproteasomes in autoinflammatory syndromes.Int Immunol. 2019 Sep 18;31(10):631-637. doi: 10.1093/intimm/dxy059. Int Immunol. 2019. PMID: 30169676 Review.
-
Proteasome-Associated Syndromes: Updates on Genetics, Clinical Manifestations, Pathogenesis, and Treatment.J Clin Immunol. 2024 Apr 5;44(4):88. doi: 10.1007/s10875-024-01692-y. J Clin Immunol. 2024. PMID: 38578475 Review.
Cited by
-
Proteostasis Perturbations and Their Roles in Causing Sterile Inflammation and Autoinflammatory Diseases.Cells. 2022 Apr 22;11(9):1422. doi: 10.3390/cells11091422. Cells. 2022. PMID: 35563729 Free PMC article. Review.
-
Establishing 20S Proteasome Genetic, Translational and Post-Translational Status from Precious Biological and Patient Samples with Top-Down MS.Cells. 2023 Mar 8;12(6):844. doi: 10.3390/cells12060844. Cells. 2023. PMID: 36980185 Free PMC article.
-
Skeletal site-specific variations in myeloid cells: insights from single-cell RNA sequencing of the mandible and femur.JBMR Plus. 2025 Apr 24;9(7):ziaf074. doi: 10.1093/jbmrpl/ziaf074. eCollection 2025 Jul. JBMR Plus. 2025. PMID: 40486046 Free PMC article.
-
Melatonin: Regulation of Biomolecular Condensates in Neurodegenerative Disorders.Antioxidants (Basel). 2021 Sep 17;10(9):1483. doi: 10.3390/antiox10091483. Antioxidants (Basel). 2021. PMID: 34573116 Free PMC article. Review.
-
Molecular dynamics simulation of wild and mutant proteasome subunit beta type 8 (PSMB8) protein: Implications for restoration of inflammation in experimental autoimmune encephalomyelitis pathogenesis.Heliyon. 2024 Dec 15;11(1):e41166. doi: 10.1016/j.heliyon.2024.e41166. eCollection 2025 Jan 15. Heliyon. 2024. PMID: 39802026 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

