Skeletal and Cardiac Muscle Disorders Caused by Mutations in Genes Encoding Intermediate Filament Proteins
- PMID: 33923914
- PMCID: PMC8073371
- DOI: 10.3390/ijms22084256
Skeletal and Cardiac Muscle Disorders Caused by Mutations in Genes Encoding Intermediate Filament Proteins
Abstract
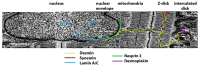
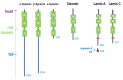
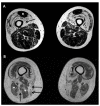
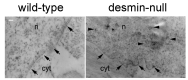
Intermediate filaments are major components of the cytoskeleton. Desmin and synemin, cytoplasmic intermediate filament proteins and A-type lamins, nuclear intermediate filament proteins, play key roles in skeletal and cardiac muscle. Desmin, encoded by the DES gene (OMIM *125660) and A-type lamins by the LMNA gene (OMIM *150330), have been involved in striated muscle disorders. Diseases include desmin-related myopathy and cardiomyopathy (desminopathy), which can be manifested with dilated, restrictive, hypertrophic, arrhythmogenic, or even left ventricular non-compaction cardiomyopathy, Emery-Dreifuss Muscular Dystrophy (EDMD2 and EDMD3, due to LMNA mutations), LMNA-related congenital Muscular Dystrophy (L-CMD) and LMNA-linked dilated cardiomyopathy with conduction system defects (CMD1A). Recently, mutations in synemin (SYNM gene, OMIM *606087) have been linked to cardiomyopathy. This review will summarize clinical and molecular aspects of desmin-, lamin- and synemin-related striated muscle disorders with focus on LMNA and DES-associated clinical entities and will suggest pathogenetic hypotheses based on the interplay of desmin and lamin A/C. In healthy muscle, such interplay is responsible for the involvement of this network in mechanosignaling, nuclear positioning and mitochondrial homeostasis, while in disease it is disturbed, leading to myocyte death and activation of inflammation and the associated secretome alterations.
Keywords: cardiomyopathy; desmin; desminopathy; lamin A/C; mechanosignaling; muscular laminopathies; nuclear positioning; secretome; synemin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Lamin-Related Congenital Muscular Dystrophy Alters Mechanical Signaling and Skeletal Muscle Growth.Int J Mol Sci. 2020 Dec 30;22(1):306. doi: 10.3390/ijms22010306. Int J Mol Sci. 2020. PMID: 33396724 Free PMC article.
-
Lamin A/C Assembly Defects in LMNA-Congenital Muscular Dystrophy Is Responsible for the Increased Severity of the Disease Compared with Emery-Dreifuss Muscular Dystrophy.Cells. 2020 Mar 31;9(4):844. doi: 10.3390/cells9040844. Cells. 2020. PMID: 32244403 Free PMC article.
-
Inhibition of extracellular signal-regulated kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins.Hum Mol Genet. 2009 Jan 15;18(2):241-7. doi: 10.1093/hmg/ddn343. Epub 2008 Oct 16. Hum Mol Genet. 2009. PMID: 18927124 Free PMC article.
-
Mutations in the LMNA gene encoding lamin A/C.Hum Mutat. 2000 Dec;16(6):451-9. doi: 10.1002/1098-1004(200012)16:6<451::AID-HUMU1>3.0.CO;2-9. Hum Mutat. 2000. PMID: 11102973 Review.
-
Laminopathies affecting skeletal and cardiac muscles: clinical and pathophysiological aspects.Acta Myol. 2005 Oct;24(2):104-9. Acta Myol. 2005. PMID: 16550926 Review.
Cited by
-
2020 Editor's Choice Articles in the "Cell Nuclei: Function, Transport and Receptors" Section.Cells. 2022 Aug 24;11(17):2625. doi: 10.3390/cells11172625. Cells. 2022. PMID: 36078033 Free PMC article.
-
Desmin-related myopathy manifested by various types of arrhythmias: a case report and literature review.J Int Med Res. 2024 Nov;52(11):3000605241291741. doi: 10.1177/03000605241291741. J Int Med Res. 2024. PMID: 39501717 Free PMC article. Review.
-
Undetected Neuromuscular Disease in Patients after Heart Transplantation.Int J Mol Sci. 2024 Jul 17;25(14):7819. doi: 10.3390/ijms25147819. Int J Mol Sci. 2024. PMID: 39063061 Free PMC article.
-
Krüpple-like factors in cardiomyopathy: emerging player and therapeutic opportunities.Front Cardiovasc Med. 2024 Mar 7;11:1342173. doi: 10.3389/fcvm.2024.1342173. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38516000 Free PMC article. Review.
-
Bi-Allelic DES Gene Variants Causing Autosomal Recessive Myofibrillar Myopathies Affecting Both Skeletal Muscles and Cardiac Function.Int J Mol Sci. 2022 Dec 14;23(24):15906. doi: 10.3390/ijms232415906. Int J Mol Sci. 2022. PMID: 36555543 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

