Polymeric Nanoparticle Delivery of Combination Therapy with Synergistic Effects in Ovarian Cancer
- PMID: 33923947
- PMCID: PMC8072532
- DOI: 10.3390/nano11041048
Polymeric Nanoparticle Delivery of Combination Therapy with Synergistic Effects in Ovarian Cancer
Abstract
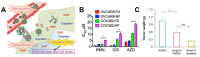
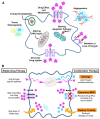
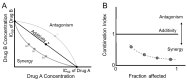
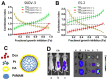
Treatment of ovarian cancer is challenging due to late stage diagnosis, acquired drug resistance mechanisms, and systemic toxicity of chemotherapeutic agents. Combination chemotherapy has the potential to enhance treatment efficacy by activation of multiple downstream pathways to overcome drug resistance and reducing required dosages. Sequence of delivery and the dosing schedule can further enhance treatment efficacy. Formulation of drug combinations into nanoparticles can further enhance treatment efficacy. Due to their versatility, polymer-based nanoparticles are an especially promising tool for clinical translation of combination therapies with tunable dosing schedules. We review polymer nanoparticle (e.g., micelles, dendrimers, and lipid nanoparticles) carriers of drug combinations formulated to treat ovarian cancer. In particular, the focus on this review is combinations of platinum and taxane agents (commonly used first line treatments for ovarian cancer) combined with other small molecule therapeutic agents. In vitro and in vivo drug potency are discussed with a focus on quantifiable synergistic effects. The effect of drug sequence and dosing schedule is examined. Computational approaches as a tool to predict synergistic drug combinations and dosing schedules as a tool for future nanoparticle design are also briefly discussed.
Keywords: cancer; combination chemotherapy; drug delivery; nanocarrier; ovarian carcinoma; polymer; synergy; therapeutic efficacy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

