Evaluation of the MeroRisk Calculator, A User-Friendly Tool to Predict the Risk of Meropenem Target Non-Attainment in Critically Ill Patients
- PMID: 33924047
- PMCID: PMC8074046
- DOI: 10.3390/antibiotics10040468
Evaluation of the MeroRisk Calculator, A User-Friendly Tool to Predict the Risk of Meropenem Target Non-Attainment in Critically Ill Patients
Abstract
Background: The MeroRisk-calculator, an easy-to-use tool to determine the risk of meropenem target non-attainment after standard dosing (1000 mg; q8h), uses a patient's creatinine clearance and the minimum inhibitory concentration (MIC) of the pathogen. In clinical practice, however, the MIC is rarely available. The objectives were to evaluate the MeroRisk-calculator and to extend risk assessment by including general pathogen sensitivity data.
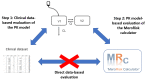
Methods: Using a clinical routine dataset (155 patients, 891 samples), a direct data-based evaluation was not feasible. Thus, in step 1, the performance of a pharmacokinetic model was determined for predicting the measured concentrations. In step 2, the PK model was used for a model-based evaluation of the MeroRisk-calculator: risk of target non-attainment was calculated using the PK model and agreement with the MeroRisk-calculator was determined by a visual and statistical (Lin's concordance correlation coefficient (CCC)) analysis for MIC values 0.125-16 mg/L. The MeroRisk-calculator was extended to include risk assessment based on EUCAST-MIC distributions and cumulative-fraction-of-response analysis.
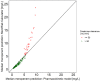
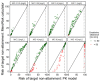
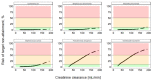
Results: Step 1 showed a negligible bias of the PK model to underpredict concentrations (-0.84 mg/L). Step 2 revealed a high level of agreement between risk of target non-attainment predictions for creatinine clearances >50 mL/min (CCC = 0.990), but considerable deviations for patients <50 mL/min. For 27% of EUCAST-listed pathogens the median cumulative-fraction-of-response for the observed patients receiving standard dosing was < 90%.
Conclusions: The MeroRisk-calculator was successfully evaluated: For patients with maintained renal function it allows a reliable and user-friendly risk assessment. The integration of pathogen-based risk assessment substantially increases the applicability of the tool.
Keywords: excel tool; individualized dosing; model-based evaluation; risk assessment.
Conflict of interest statement
C.K. and W.H. report grants from an industry consortium (AbbVie Deutschland GmbH & Co. KG, AstraZeneca, Boehringer Ingelheim Pharma GmbH & Co. KG, Grünenthal GmbH, F. Hoffmann-La Roche Ltd., Merck KGaA and SANOFI) for the PharMetrX program. CK reports grants from the Innovative Medicines Initiative-Joint Undertaking (“DDMoRe”), Diurnal Ltd., the Federal Ministry of Education and Research within the Joint Programming Initiative on Antimicrobial Resistance Initiative (JPIAMR) and the European Commission within in the Horizon 2020 framework programme (“FAIR”), all outside the submitted work.
Figures
Similar articles
-
Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: a prospective observational study.Crit Care. 2017 Oct 21;21(1):263. doi: 10.1186/s13054-017-1829-4. Crit Care. 2017. PMID: 29058601 Free PMC article. Clinical Trial.
-
Meropenem Pharmacokinetics and Target Attainment in Critically Ill Patients.Infect Drug Resist. 2023 Jun 21;16:3989-3997. doi: 10.2147/IDR.S408572. eCollection 2023. Infect Drug Resist. 2023. PMID: 37366501 Free PMC article.
-
Pharmacokinetic-Pharmacodynamic Target Attainment Analyses as Support for Meropenem-Vaborbactam Dosing Regimens and Susceptibility Breakpoints.Antimicrob Agents Chemother. 2022 Dec 20;66(12):e0213021. doi: 10.1128/aac.02130-21. Epub 2022 Nov 14. Antimicrob Agents Chemother. 2022. PMID: 36374023 Free PMC article.
-
Meropenem for the Pharmacological Treatment of Severe Infections in Critically Ill Pediatric Patients: Breakthrough Standard Treatment Strategies Based on PK/PD.Curr Drug Metab. 2023;24(1):5-15. doi: 10.2174/1389200224666230325121729. Curr Drug Metab. 2023. PMID: 36974414 Review.
-
Optimal Meropenem Dosing Regimens in Patients Undergoing Continuous Renal Replacement Therapy: Systematic Review and Monte Carlo Simulations.Blood Purif. 2023;52(6):503-515. doi: 10.1159/000529694. Epub 2023 May 5. Blood Purif. 2023. PMID: 37231811
Cited by
-
Comparative Plasma and Interstitial Tissue Fluid Pharmacokinetics of Meropenem Demonstrate the Need for Increasing Dose and Infusion Duration in Obese and Non-obese Patients.Clin Pharmacokinet. 2022 May;61(5):655-672. doi: 10.1007/s40262-021-01070-6. Epub 2021 Dec 11. Clin Pharmacokinet. 2022. PMID: 34894344 Free PMC article. Clinical Trial.
-
Optimal loading dose of meropenem before continuous infusion in critically ill patients: a simulation study.Sci Rep. 2021 Aug 26;11(1):17211. doi: 10.1038/s41598-021-96744-3. Sci Rep. 2021. PMID: 34446780 Free PMC article.
-
Combination of Pharmacokinetic and Pathogen Susceptibility Information To Optimize Meropenem Treatment of Gram-Negative Infections in Critically Ill Patients.Antimicrob Agents Chemother. 2022 Feb 15;66(2):e0183121. doi: 10.1128/AAC.01831-21. Epub 2021 Dec 6. Antimicrob Agents Chemother. 2022. PMID: 34871092 Free PMC article.
-
Population Pharmacokinetics of Meropenem in Critically Ill Korean Patients and Effects of Extracorporeal Membrane Oxygenation.Pharmaceutics. 2021 Nov 4;13(11):1861. doi: 10.3390/pharmaceutics13111861. Pharmaceutics. 2021. PMID: 34834278 Free PMC article.
References
-
- Engel C., Brunkhorst F.M., Bone H.-G., Brunkhorst R., Gerlach H., Grond S., Gruendling M., Huhle G., Jaschinski U., John S., et al. Epidemiology of Sepsis in Germany: Results from a National Prospective Multicenter Study. Intensiv. Care Med. 2007;33:606–618. doi: 10.1007/s00134-006-0517-7. - DOI - PubMed
-
- Kumar A., Roberts D., Wood K.E., Light B., Parrillo J.E., Sharma S., Suppes R., Feinstein D., Zanotti S., Taiberg L., et al. Duration of Hypotension before Initiation of Effective Antimicrobial Therapy Is the Critical Determinant of Survival in Human Septic Shock. Crit. Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. - DOI - PubMed
-
- Ferrer R., Martin-Loeches I., Phillips G., Osborn T.M., Townsend S., Dellinger R.P., Artigas A., Schorr C., Levy M.M. Empiric Antibiotic Treatment Reduces Mortality in Severe Sepsis and Septic Shock from the First Hour: Results from a Guideline-Based Performance Improvement Program. Crit. Care Med. 2014;42:1749–1755. doi: 10.1097/CCM.0000000000000330. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

