Revisiting the Impact of Local Leptin Signaling in Folliculogenesis and Oocyte Maturation in Obese Mothers
- PMID: 33924072
- PMCID: PMC8074257
- DOI: 10.3390/ijms22084270
Revisiting the Impact of Local Leptin Signaling in Folliculogenesis and Oocyte Maturation in Obese Mothers
Abstract
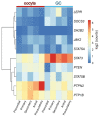
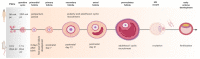
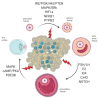
The complex nature of folliculogenesis regulation accounts for its susceptibility to maternal physiological fitness. In obese mothers, progressive expansion of adipose tissue culminates with severe hyperestrogenism and hyperleptinemia with detrimental effects for ovarian performance. Indeed, maternal obesity is associated with the establishment of ovarian leptin resistance. This review summarizes current knowledge on potential effects of impaired leptin signaling throughout folliculogenesis and oocyte developmental competence in mice and women.
Keywords: folliculogenesis; leptin; obesity; oocyte; ovary.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Yang X., Wu L.L., Chura L.R., Liang X., Lane M., Norman R.J., Robker R.L. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil. Steril. 2012;97:1438–1443. doi: 10.1016/j.fertnstert.2012.02.034. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

