Radiological and Clinical Efficacy of Intra-Arterial 90Y-DOTATATE in Patients with Unresectable, Progressive, Liver Dominant Neuroendocrine Neoplasms
- PMID: 33924160
- PMCID: PMC8074370
- DOI: 10.3390/jcm10081794
Radiological and Clinical Efficacy of Intra-Arterial 90Y-DOTATATE in Patients with Unresectable, Progressive, Liver Dominant Neuroendocrine Neoplasms
Abstract
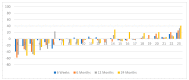
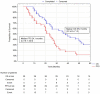
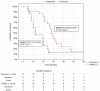
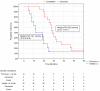
This study was performed to determine if intra-arterial (i.a.) administration of 90Y DOTATATE can provide an effective and safe alternative to the accepted standard for i.v. of peptide receptor radionuclide therapy (PRRT) in liver-dominant metastases of gastrointestinal pancreatic neuroendocrine neoplasm (GEP-NEN). A single site, prospective, preliminary case series study included 39 patients with histologically proven liver-dominant NEN. PRRT in the form of 1.15GBq 90Y DOTATATE was given selectively into the liver via radiological catheterization of the hepatic artery, up to four times. The endpoint was radiological response (RECIST). Secondary endpoints assessed clinical well-being post-treatment, progression-free survival (PFS), overall survival (OS), and toxicity. Partial response (PR) was noted in 13% of subjects six weeks post-therapy, increasing to 24% at six months and dropping to 13% at 36 months. Disease progression (DP) was not seen at six weeks, was 5% at six months, and 47% at 36 months. Clinical response based on PS seen in 74% of patients at six weeks, 69% at six months, and 39% at 36 months had PFS and OS, respectively, of 22.7 months and 38.2 months. There was no difference in OS/PFS between those with RECIST PR and SD. One patient had significant toxicity (3%). Use of i.a. PRRT appears to be safe and effective in treating patients with liver-dominant NEN. In addition, the best OS (51 vs. 22 months) was seen when i.a. was used as an upfront treatment of bulky GEP-NEN liver metastases and not after i.v. 90Y DOTATATE. The use of i.a. 90Y DOTATATE PRRT appears to be safe and effective in treating patients with liver-dominant NEN.
Keywords: 90Y-DOTATATE; OS; PFS; PRRT-intra-arterial (i.a.); neuroendocrine neoplasms; radiological and clinical response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ramage J.K., Ahmed A., Ardill J., Bax N., Breen D.J., Caplin M.E., Corrie P., Davar J., Davies A.H., Lewington V., et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs) Gut. 2011;61:6–32. doi: 10.1136/gutjnl-2011-300831. - DOI - PMC - PubMed
-
- Yao J.C., Fazio N., Singh S., Buzzoni R., Carnaghi C., Wolin E., Tomasek J., Raderer M., Lahner H., Voi M., et al. Everolimus ofr the treatment of advanced non-functional neuroendocrine tumours of the lung or gastrointestinal tract (Radiant-4): A randomized, placebo-controlled phase 3 study. Lancet. 2016;387:968–977. doi: 10.1016/S0140-6736(15)00817-X. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

