Flame Retardants-Mediated Interferon Signaling in the Pathogenesis of Nonalcoholic Fatty Liver Disease
- PMID: 33924165
- PMCID: PMC8074384
- DOI: 10.3390/ijms22084282
Flame Retardants-Mediated Interferon Signaling in the Pathogenesis of Nonalcoholic Fatty Liver Disease
Abstract
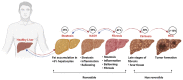
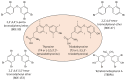
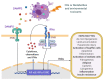
Nonalcoholic fatty liver disease (NAFLD) is a growing concern worldwide, affecting 25% of the global population. NAFLD is a multifactorial disease with a broad spectrum of pathology includes steatosis, which gradually progresses to a more severe condition such as nonalcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and eventually leads to hepatic cancer. Several risk factors, including exposure to environmental toxicants, are involved in the development and progression of NAFLD. Environmental factors may promote the development and progression of NAFLD by various biological alterations, including mitochondrial dysfunction, reactive oxygen species production, nuclear receptors dysregulation, and interference in inflammatory and immune-mediated signaling. Moreover, environmental contaminants can influence immune responses by impairing the immune system's components and, ultimately, disease susceptibility. Flame retardants (FRs) are anthropogenic chemicals or mixtures that are being used to inhibit or delay the spread of fire. FRs have been employed in several household and outdoor products; therefore, human exposure is unavoidable. In this review, we summarized the potential mechanisms of FRs-associated immune and inflammatory signaling and their possible contribution to the development and progression of NAFLD, with an emphasis on FRs-mediated interferon signaling. Knowledge gaps are identified, and emerging pharmacotherapeutic molecules targeting the immune and inflammatory signaling for NAFLD are also discussed.
Keywords: cytokines; flame retardants; interferon; metabolic disruption; metabolism-disrupting chemicals; nonalcoholic fatty liver disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

