Astrocyte Activation in Neurovascular Damage and Repair Following Ischaemic Stroke
- PMID: 33924191
- PMCID: PMC8074612
- DOI: 10.3390/ijms22084280
Astrocyte Activation in Neurovascular Damage and Repair Following Ischaemic Stroke
Abstract
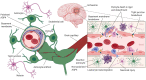
Transient or permanent loss of tissue perfusion due to ischaemic stroke can lead to damage to the neurovasculature, and disrupt brain homeostasis, causing long-term motor and cognitive deficits. Despite promising pre-clinical studies, clinically approved neuroprotective therapies are lacking. Most studies have focused on neurons while ignoring the important roles of other cells of the neurovascular unit, such as astrocytes and pericytes. Astrocytes are important for the development and maintenance of the blood-brain barrier, brain homeostasis, structural support, control of cerebral blood flow and secretion of neuroprotective factors. Emerging data suggest that astrocyte activation exerts both beneficial and detrimental effects following ischaemic stroke. Activated astrocytes provide neuroprotection and contribute to neurorestoration, but also secrete inflammatory modulators, leading to aggravation of the ischaemic lesion. Astrocytes are more resistant than other cell types to stroke pathology, and exert a regulative effect in response to ischaemia. These roles of astrocytes following ischaemic stroke remain incompletely understood, though they represent an appealing target for neurovascular protection following stroke. In this review, we summarise the astrocytic contributions to neurovascular damage and repair following ischaemic stroke, and explore mechanisms of neuroprotection that promote revascularisation and neurorestoration, which may be targeted for developing novel therapies for ischaemic stroke.
Keywords: astrocyte; neuroinflammation; neuroprotection; neurorestoration; neurotoxicity; neurovascular; secretion; stroke.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gribkoff V.K., Starrett J.E., Jr., Dworetzky S.I., Hewawasam P., Boissard C.G., Cook D.A., Frantz S.W., Heman K., Hibbard J.R., Huston K., et al. Targeting acute ischemic stroke with a calcium-sensitive opener of maxi-K potassium channels. Nat. Med. 2001;7:471–477. doi: 10.1038/86546. - DOI - PubMed
-
- Campbell B.C.V., Ma H., Ringleb P.A., Parsons M.W., Churilov L., Bendszus M., Levi C.R., Hsu C., Kleinig T.J., Fatar M., et al. Extending thrombolysis to 4·5-9 h and wake-up stroke using perfusion imaging: A systematic review and meta-analysis of individual patient data. Lancet. 2019;394:139–147. doi: 10.1016/S0140-6736(19)31053-0. - DOI - PubMed
-
- Campbell B.C.V., Donnan G.A., Lees K.R., Hacke W., Khatri P., Hill M.D., Goyal M., Mitchell P.J., Saver J.L., Diener H.C., et al. Endovascular stent thrombectomy: The new standard of care for large vessel ischaemic Stroke. Lancet Neurol. 2015;14:846–854. doi: 10.1016/S1474-4422(15)00140-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

