In Vitro Antibacterial, Anti-Adhesive and Anti-Biofilm Activities of Krameria lappacea (Dombey) Burdet & B.B. Simpson Root Extract against Methicillin-Resistant Staphylococcus aureus Strains
- PMID: 33924336
- PMCID: PMC8069196
- DOI: 10.3390/antibiotics10040428
In Vitro Antibacterial, Anti-Adhesive and Anti-Biofilm Activities of Krameria lappacea (Dombey) Burdet & B.B. Simpson Root Extract against Methicillin-Resistant Staphylococcus aureus Strains
Erratum in
-
Correction: Genovese et al. In Vitro Antibacterial, Anti-Adhesive and Anti-Biofilm Activities of Krameria lappacea (Dombey) Burdet & B.B. Simpson Root Extract against Methicillin-Resistant Staphylococcus aureus Strains. Antibiotics 2021, 10, 428.Antibiotics (Basel). 2021 Jun 30;10(7):799. doi: 10.3390/antibiotics10070799. Antibiotics (Basel). 2021. PMID: 34209470 Free PMC article.
Abstract
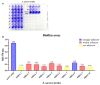
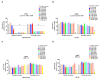
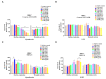
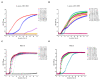
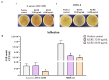
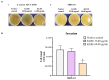
Methicillin-resistant Staphylococcus aureus (MRSA) represents a serious threat to public health, due to its large variety of pathogenetic mechanisms. Accordingly, the present study aimed to investigate the anti-MRSA activities of Krameria lappacea, a medicinal plant native to South America. Through Ultra-High-Performance Liquid Chromatography coupled with High-Resolution Mass spectrometry, we analyzed the chemical composition of Krameria lappacea root extract (KLRE). The antibacterial activity of KLRE was determined by the broth microdilution method, also including the minimum biofilm inhibitory concentration and minimum biofilm eradication concentration. Besides, we evaluated the effect on adhesion and invasion of human lung carcinoma A549 cell line by MRSA strains. The obtained results revealed an interesting antimicrobial action of this extract, which efficiently inhibit the growth, biofilm formation, adhesion and invasion of MRSA strains. Furthermore, the chemical analysis revealed the presence in the extract of several flavonoid compounds and type-A and type-B proanthocyanidins, which are known for their anti-adhesive effects. Taken together, our findings showed an interesting antimicrobial activity of KLRE, giving an important contribution to the current knowledge on the biological activities of this plant.
Keywords: A549 cells; Krameria lappacea; adhesion and invasion; biofilm; methicillin-resistant Staphylococcus aureus; proanthocyanidins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Yang E.S., Tan J., Eells S., Rieg G., Tagudar G., Miller L.G. Body site colonization in patients with community-associated methicillin-resistant Staphylococcus aureus and other types of S. aureus skin infections. Clin. Microbiol. Infect. 2010;16:425–431. doi: 10.1111/j.1469-0691.2009.02836.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

